Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis
- PMID: 19513120
- PMCID: PMC2688760
- DOI: 10.1371/journal.pone.0005844
Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis
Abstract
Background: Obesity-associated inflammation is of critical importance in the development of insulin resistance and non-alcoholic fatty liver disease. Since the cannabinoid receptor CB2 regulates innate immunity, the aim of the present study was to investigate its role in obesity-induced inflammation, insulin resistance and fatty liver.
Methodology: Murine obesity models included genetically leptin-deficient ob/ob mice and wild type (WT) mice fed a high fat diet (HFD), that were compared to their lean counterparts. Animals were treated with pharmacological modulators of CB2 receptors. Experiments were also performed in mice knock-out for CB2 receptors (Cnr2 -/-).
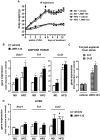
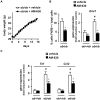
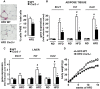
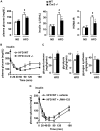
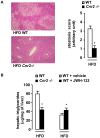
Principal findings: In both HFD-fed WT mice and ob/ob mice, Cnr2 expression underwent a marked induction in the stromal vascular fraction of epididymal adipose tissue that correlated with increased fat inflammation. Treatment with the CB2 agonist JWH-133 potentiated adipose tissue inflammation in HFD-fed WT mice. Moreover, cultured fat pads isolated from ob/ob mice displayed increased Tnf and Ccl2 expression upon exposure to JWH-133. In keeping, genetic or pharmacological inactivation of CB2 receptors decreased adipose tissue macrophage infiltration associated with obesity, and reduced inductions of Tnf and Ccl2 expressions. In the liver of obese mice, Cnr2 mRNA was only weakly induced, and CB2 receptors moderately contributed to liver inflammation. HFD-induced insulin resistance increased in response to JWH-133 and reduced in Cnr2 -/- mice. Finally, HFD-induced hepatic steatosis was enhanced in WT mice treated with JWH-133 and blunted in Cnr2 -/- mice.
Conclusion/significance: These data unravel a previously unrecognized contribution of CB2 receptors to obesity-associated inflammation, insulin resistance and non-alcoholic fatty liver disease, and suggest that CB2 receptor antagonists may open a new therapeutic approach for the management of obesity-associated metabolic disorders.
Conflict of interest statement
Figures

References
-
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. - PubMed
-
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. - PubMed
-
- Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. - PubMed
-
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

