Sugar-mediated semidian oscillation of gene expression in the cassava storage root regulates starch synthesis
- PMID: 19513234
- PMCID: PMC2634422
- DOI: 10.4161/psb.3.7.5715
Sugar-mediated semidian oscillation of gene expression in the cassava storage root regulates starch synthesis
Abstract
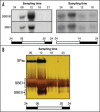
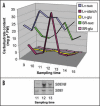
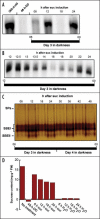
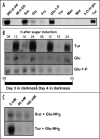
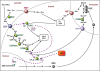
Starch branching enzyme (SBE) activity in the cassava storage root exhibited a diurnal fluctuation, dictated by a transcriptional oscillation of the corresponding SBE genes. The peak of SBE activity coincided with the onset of sucrose accumulation in the storage, and we conclude that the oscillatory mechanism keeps the starch synthetic apparatus in the storage root sink in tune with the flux of sucrose from the photosynthetic source. When storage roots were uncoupled from the source, SBE expression could be effectively induced by exogenous sucrose. Turanose, a sucrose isomer that cannot be metabolized by plants, mimicked the effect of sucrose, demonstrating that downstream metabolism of sucrose was not necessary for signal transmission. Also glucose and glucose-1-P induced SBE expression. Interestingly, induction by sucrose, turanose and glucose but not glucose-1-P sustained an overt semidian (12-h) oscillation in SBE expression and was sensitive to the hexokinase (HXK) inhibitor glucosamine. These results suggest a pivotal regulatory role for HXK during starch synthesis. Abscisic acid (ABA) was another potent inducer of SBE expression. Induction by ABA was similar to that of glucose-1-P in that it bypassed the semidian oscillator. Both the sugar and ABA signaling cascades were disrupted by okadaic acid, a protein phosphatase inhibitor. Based on these findings, we propose a model for sugar signaling in regulation of starch synthesis in the cassava storage root.
Keywords: SBE; cassava; semidian oscillation; starch synthesis; sugar signaling.
Figures

References
-
- Jansson C. Sugar signaling mutants in Arabidopsis. In: Esser K, et al., editors. Progress in Botany. Berlin: Springer Verlag; 2004. pp. 42–52.
-
- Rolland F, Baena Gonzalez E, Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:675–709. - PubMed
-
- Sheen J, Zhou L, Jang JC. Sugars as signaling molecules. Curr Opinions Plant Biol. 1999;2:410–418. - PubMed
-
- Smeekens S. Sugar-Induced Signal Transduction in Plants. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. - PubMed
-
- Sun C, Olsson H, Mangelsen E, Höglund AS, Jansson C. Antisense ODN inhibition as a potent strategy in plant biology: identification of SUSIBA2 as a transcriptional activator in plant sugar signaling. Plant J. 2005;44:128–138. - PubMed
