The role of hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology
- PMID: 19515211
- PMCID: PMC2814768
- DOI: 10.1111/j.1743-6109.2009.01349.x
The role of hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology
Abstract
Introduction: Sonic hedgehog (SHH) is an essential regulator of smooth muscle apoptosis in the penis that has significant clinical potential as a therapy to suppress post-prostatectomy apoptosis, an underlying cause of erectile dysfunction (ED). Thus an understanding of how SHH signaling is regulated in the adult penis is essential to move the field of ED research forward and to develop new treatment strategies. We propose that hedgehog-interacting protein (HIP), which has been shown to bind SHH protein and to play a role in SHH regulation during embryogenesis of other organs, is a critical regulator of SHH signaling, penile morphology, and apoptosis induction.
Aims: We have examined HIP signaling in the penis and cavernous nerve (CN) during postnatal differentiation of the penis, in CN-injured, and a diabetic model of ED.
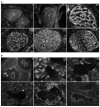
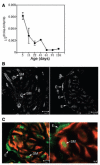
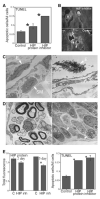
Methods: HIP localization/abundance and RNA abundance were examined by immunohistochemical (IHC) analysis and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) in Sprague-Dawley rats between the ages of 7 and 92 days old, in CN-injured Sprague-Dawley rats and in BioBreeding/Worcester diabetic rats. HIP signaling was perturbed in the pelvic ganglia and in the penis and TUNEL assay was performed in the penis. CN tie, lidocaine, and anti-kinesin experiments were performed to examine HIP signaling in the CN and penis.
Results: In this study we are the first to demonstrate that HIP undergoes anterograde transport to the penis via the CN, that HIP perturbation in the pelvic ganglia or the penis induces apoptosis, and that HIP plays a role in maintaining CN integrity, penile morphology, and SHH abundance.
Conclusions: These studies are significant because they show HIP involvement in cross-talk (signaling) between the pelvic ganglia and penis, which is integral for maintenance of penile morphology and they suggest a mechanism of how nerves may regulate target organ morphology and function.
Figures


Similar articles
-
Sonic hedgehog suppresses penile remodeling after cavernous nerve injury and sustains long-term normal penis morphology.J Sex Med. 2024 Oct 31;21(11):986-993. doi: 10.1093/jsxmed/qdae116. J Sex Med. 2024. PMID: 39279183
-
Sonic hedgehog delivery from self-assembled nanofiber hydrogels reduces the fibrotic response in models of erectile dysfunction.Acta Biomater. 2016 Mar 1;32:89-99. doi: 10.1016/j.actbio.2016.01.014. Epub 2016 Jan 14. Acta Biomater. 2016. PMID: 26776147 Free PMC article.
-
Neural influences on sonic hedgehog and apoptosis in the rat penis.Biol Reprod. 2008 May;78(5):947-56. doi: 10.1095/biolreprod.107.064766. Epub 2008 Feb 6. Biol Reprod. 2008. PMID: 18256331 Free PMC article.
-
Sonic hedgehog, apoptosis, and the penis.J Sex Med. 2009 Mar;6 Suppl 3(Suppl 3):334-9. doi: 10.1111/j.1743-6109.2008.01192.x. J Sex Med. 2009. PMID: 19267857 Free PMC article. Review.
-
Sonic hedgehog, the penis and erectile dysfunction: a review of sonic hedgehog signaling in the penis.Curr Pharm Des. 2005;11(31):4011-27. doi: 10.2174/138161205774913408. Curr Pharm Des. 2005. PMID: 16378507 Review.
Cited by
-
Molecular pathogenesis and treatment of cavernous nerve injury-induced erectile dysfunction: A narrative review.Front Physiol. 2022 Oct 6;13:1029650. doi: 10.3389/fphys.2022.1029650. eCollection 2022. Front Physiol. 2022. PMID: 36277218 Free PMC article. Review.
-
Peptide amphiphile nanofiber delivery of sonic hedgehog protein to reduce smooth muscle apoptosis in the penis after cavernous nerve resection.J Sex Med. 2011 Jan;8(1):78-89. doi: 10.1111/j.1743-6109.2010.02001.x. Epub 2010 Aug 30. J Sex Med. 2011. PMID: 20807324 Free PMC article.
-
Sonic hedgehog is neuroprotective in the cavernous nerve with crush injury.J Sex Med. 2013 May;10(5):1240-50. doi: 10.1111/j.1743-6109.2012.02930.x. Epub 2012 Sep 20. J Sex Med. 2013. PMID: 22994531 Free PMC article.
-
Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers.Biomaterials. 2011 Feb;32(4):1091-101. doi: 10.1016/j.biomaterials.2010.10.003. Epub 2010 Oct 23. Biomaterials. 2011. PMID: 20971506 Free PMC article.
-
Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction.Nanomedicine. 2017 Jan;13(1):95-101. doi: 10.1016/j.nano.2016.08.032. Epub 2016 Sep 6. Nanomedicine. 2017. PMID: 27609775 Free PMC article.
References
-
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. - PubMed
-
- Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D. Prevalence of erectile dysfunction among young adults: Results of a large-scale survey. J Sex Med. 2004;1:284–91. - PubMed
-
- Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

