Comparison of oral and vaginal metronidazole for treatment of bacterial vaginosis in pregnancy: impact on fastidious bacteria
- PMID: 19515236
- PMCID: PMC2703644
- DOI: 10.1186/1471-2334-9-89
Comparison of oral and vaginal metronidazole for treatment of bacterial vaginosis in pregnancy: impact on fastidious bacteria
Abstract
Background: Bacterial vaginosis (BV) is a common condition that is associated with preterm birth and acquisition of complex communities of vaginal bacteria that include several fastidious species. Treatment of BV in pregnancy has mixed effects on the risk of preterm delivery, which some hypothesize is due to variable antibiotic efficacy for the fastidious bacteria. Both oral and intravaginal metronidazole can be used to treat bacterial vaginosis in pregnancy, but little is known about the impact of different routes of antibiotic administration on concentrations of fastidious vaginal bacteria.
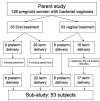
Methods: This was a sub-study of a larger randomized trial of oral versus vaginal metronidazole for treatment of BV in pregnancy. Fifty-three women were evaluated, including 30 women who received oral metronidazole and 23 who received intravaginal metronidazole. Bacterial taxon-specific quantitative PCR assays were used to measure concentrations of bacterial vaginosis associated bacterium (BVAB) 1, 2, and 3, Gardnerella vaginalis, Atopobium species, Leptotrichia/Sneathia species, Megasphaera species, and Lactobacillus crispatus before and after antibiotic treatment.
Results: Concentrations of Leptotrichia and Sneathia spp. and the fastidious Clostridia-like bacterium designated BVAB1 decreased significantly with oral (p = .002, p = .02) but not vaginal therapy (p = .141, p = .126). The fastidious bacterium BVAB3 did not significantly decrease with either treatment. Concentrations of Atopobium spp., reportedly resistant to metronidazole in vitro, dropped significantly with oral (p = .002) and vaginal (p = .001) treatment. There was no significant difference in the magnitude of change in bacterial concentrations between oral and vaginal treatment arms for any of the bacterial species. Lactobacillus crispatus concentrations did not change.
Conclusion: Both oral and vaginal metronidazole therapy in pregnant women result in a significant decrease in concentrations of most BV-associated anaerobic bacteria, with the exception that Leptotrichia, Sneathia and BVAB1 do not significantly decrease with vaginal metronidazole therapy. These data suggest that the route of antibiotic administration has a minor impact on bacterial eradication in pregnant women with BV.
Trial registration: This trial is registered with ClinicalTrials.gov, number NCT00153517.
Figures
References
-
- Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstetrics and gynecology. 2007;109(1):114–120. - PubMed
-
- Kurki T, Sivonen A, Renkonen OV, Savia E, Ylikorkala O. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstetrics and gynecology. 1992;80(2):173–177. - PubMed
-
- Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. The Journal of infectious diseases. 2006;193(11):1478–1486. doi: 10.1086/503780. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

