Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I
- PMID: 19515907
- PMCID: PMC2723061
- DOI: 10.1523/JNEUROSCI.1341-09.2009
Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I
Abstract
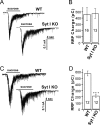
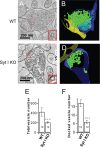
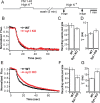
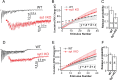
Synaptotagmin-I (syt-I) is required for rapid neurotransmitter release in mouse hippocampal neurons. However, contradictory results have been reported regarding evoked and spontaneous secretion from syt-I knock-out (KO) neurons. Here, we compared synaptic transmission in two different hippocampal neuron preparations: autaptic cultures in which a single isolated cell innervates itself, and dissociated mass cultures in which individual cells are innervated by neighboring cells. In autaptic cultures, the total extent of evoked release, size of readily releasable pool of synaptic vesicles, and release probability were unchanged in syt-I KO neurons. In contrast, in cultures containing multiple interconnected neurons, total evoked release, the number of docked vesicles, and release probability, were significantly reduced in syt-I KO neurons. Using a micronetwork system in which we varied the number of cells on an island, we found that the frequency of spontaneous synaptic vesicle fusion events (minis) was unchanged in syt-I KO neurons when two or fewer cells were present on an island. However, in micronetworks composed of three or more neurons, mini frequency was increased threefold to fivefold in syt-I KO neurons compared with wild type. Moreover, interneuronal synapses exhibited higher rates of spontaneous release than autaptic synapses. This higher rate was attributable to an increase in release probability because excitatory hippocampal neurons in micronetworks formed a set number of synapses per cell regardless of the number of connected neurons. Thus, aspects of synaptic transmission differ between autaptic and dissociated cultures, and the synaptic transmission phenotype, resulting from loss of syt-I, is dictated by the connectivity of neurons.
Figures

Similar articles
-
Interactions Between SNAP-25 and Synaptotagmin-1 Are Involved in Vesicle Priming, Clamping Spontaneous and Stimulating Evoked Neurotransmission.J Neurosci. 2016 Nov 23;36(47):11865-11880. doi: 10.1523/JNEUROSCI.1011-16.2016. J Neurosci. 2016. PMID: 27881774 Free PMC article.
-
Ca2+ sensor proteins in spontaneous release and synaptic plasticity: Limited contribution of Doc2c, rabphilin-3a and synaptotagmin 7 in hippocampal glutamatergic neurons.Mol Cell Neurosci. 2021 Apr;112:103613. doi: 10.1016/j.mcn.2021.103613. Epub 2021 Mar 19. Mol Cell Neurosci. 2021. PMID: 33753311
-
Loss of synaptotagmin IV results in a reduction in synaptic vesicles and a distortion of the Golgi structure in cultured hippocampal neurons.Neuroscience. 2010 Apr 28;167(1):135-42. doi: 10.1016/j.neuroscience.2010.01.056. Epub 2010 Feb 4. Neuroscience. 2010. PMID: 20138128 Free PMC article.
-
Roles of SNARE proteins and synaptotagmin I in synaptic transmission: studies at the Drosophila neuromuscular synapse.Neurosignals. 2003 Jan-Feb;12(1):13-30. doi: 10.1159/000068912. Neurosignals. 2003. PMID: 12624525 Review.
-
The role of ribbons at sensory synapses.Neuroscientist. 2009 Aug;15(4):380-91. doi: 10.1177/1073858408331373. Epub 2009 Mar 4. Neuroscientist. 2009. PMID: 19264728 Free PMC article. Review.
Cited by
-
Glycosylation is dispensable for sorting of synaptotagmin 1 but is critical for targeting of SV2 and synaptophysin to recycling synaptic vesicles.J Biol Chem. 2012 Oct 12;287(42):35658-35668. doi: 10.1074/jbc.M112.398883. Epub 2012 Aug 20. J Biol Chem. 2012. PMID: 22908222 Free PMC article.
-
Deconstructing Synaptotagmin-1's Distinct Roles in Synaptic Vesicle Priming and Neurotransmitter Release.J Neurosci. 2022 Apr 6;42(14):2856-2871. doi: 10.1523/JNEUROSCI.1945-21.2022. Epub 2022 Feb 22. J Neurosci. 2022. PMID: 35193927 Free PMC article.
-
Differential Regulation of Synaptic Vesicle Tethering and Docking by UNC-18 and TOM-1.Front Synaptic Neurosci. 2010 Oct 5;2:141. doi: 10.3389/fnsyn.2010.00141. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423527 Free PMC article.
-
Autaptic Cultures: Methods and Applications.Front Synaptic Neurosci. 2020 Apr 30;12:18. doi: 10.3389/fnsyn.2020.00018. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32425765 Free PMC article.
-
Synaptotagmin-7 is an asynchronous calcium sensor for synaptic transmission in neurons expressing SNAP-23.PLoS One. 2014 Nov 25;9(11):e114033. doi: 10.1371/journal.pone.0114033. eCollection 2014. PLoS One. 2014. PMID: 25422940 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
