A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire
- PMID: 19515917
- PMCID: PMC2758634
- DOI: 10.1523/JNEUROSCI.6044-08.2009
A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire
Abstract
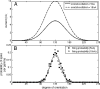
The role of gamma oscillations in producing synchronized firing of groups of principal cells is well known. Here, we argue that gamma oscillations have a second function: they select which principal cells fire. This selection process occurs through the interaction of excitation with gamma frequency feedback inhibition. We sought to understand the rules that govern this process. One possibility is that a constant fraction of cells fire. Our analysis shows, however, that the fraction is not robust because it depends on the distribution of excitation to different cells. A robust description is termed E%-max: cells fire if they have suprathreshold excitation (E) within E% of the cell that has maximum excitation. The value of E%-max is approximated by the ratio of the delay of feedback inhibition to the membrane time constant. From measured values, we estimate that E%-max is 5-15%. Thus, an E%-max winner-take-all process can discriminate between groups of cells that have only small differences in excitation. To test the utility of this framework, we analyzed the role of oscillations in V1, one of the few systems in which both spiking and intracellular excitation have been directly measured. We show that an E%-max winner-take-all process provides a simple explanation for why the orientation tuning of firing is narrower than that of the excitatory input and why this difference is not affected by increasing excitation. Because gamma oscillations occur in many brain regions, the framework we have developed for understanding the second function of gamma is likely to have wide applicability.
Figures

References
-
- Anderson JS, Lampl I, Gillespie DC, Ferster D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science. 2000;290:1968–1972. - PubMed
-
- Andrade R. Cell excitation enhances muscarinic cholinergic responses in rat association cortex. Brain Res. 1991;548:81–93. - PubMed
-
- Araneda R, Andrade R. 5-Hydroxytryptamine 2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. - PubMed
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
