Shedding light on serpent sight: the visual pigments of henophidian snakes
- PMID: 19515920
- PMCID: PMC6665397
- DOI: 10.1523/JNEUROSCI.0517-09.2009
Shedding light on serpent sight: the visual pigments of henophidian snakes
Abstract
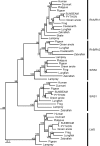
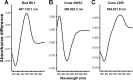
The biologist Gordon Walls proposed his "transmutation" theory through the 1930s and the 1940s to explain cone-like morphology of rods (and vice versa) in the duplex retinas of modern-day reptiles, with snakes regarded as the epitome of his hypothesis. Despite Walls' interest, the visual system of reptiles, and in particular snakes, has been widely neglected in favor of studies of fishes and mammals. By analyzing the visual pigments of two henophidian snakes, Xenopeltis unicolor and Python regius, we show that both species express two cone opsins, an ultraviolet-sensitive short-wavelength-sensitive 1 (SWS1) (lambda(max) = 361 nm) pigment and a long-wavelength-sensitive (LWS) (lambda(max) = 550 nm) pigment, providing the potential for dichromatic color vision. They also possess rod photoreceptors which express the usual rod opsin (Rh1) pigment with a lambda(max) at 497 nm. This is the first molecular study of the visual pigments expressed in the photoreceptors of any snake species. The presence of a duplex retina and the characterization of LWS, SWS1, and Rh1 visual pigments in henophidian snakes implies that "lower" snakes do not provide support for Walls' transmutation theory, unlike some "higher" (caenophidian) snakes and other reptiles, such as geckos. More data from other snake lineages will be required to test this hypothesis further.
Figures



Similar articles
-
Multiple rod-cone and cone-rod photoreceptor transmutations in snakes: evidence from visual opsin gene expression.Proc Biol Sci. 2016 Jan 27;283(1823):20152624. doi: 10.1098/rspb.2015.2624. Epub 2016 Jan 27. Proc Biol Sci. 2016. PMID: 26817768 Free PMC article.
-
Visual Pigments, Ocular Filters and the Evolution of Snake Vision.Mol Biol Evol. 2016 Oct;33(10):2483-95. doi: 10.1093/molbev/msw148. Epub 2016 Aug 16. Mol Biol Evol. 2016. PMID: 27535583
-
Adaptations and evolutionary trajectories of the snake rod and cone photoreceptors.Semin Cell Dev Biol. 2020 Oct;106:86-93. doi: 10.1016/j.semcdb.2020.04.004. Epub 2020 Apr 28. Semin Cell Dev Biol. 2020. PMID: 32359892 Review.
-
Visual system evolution and the nature of the ancestral snake.J Evol Biol. 2015 Jul;28(7):1309-20. doi: 10.1111/jeb.12663. Epub 2015 Jun 16. J Evol Biol. 2015. PMID: 26012745
-
The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies.Cell Mol Life Sci. 2007 Nov;64(22):2917-32. doi: 10.1007/s00018-007-7253-1. Cell Mol Life Sci. 2007. PMID: 17726575 Free PMC article. Review.
Cited by
-
A dune with a view: the eyes of a neotropical fossorial lizard.Front Zool. 2019 Jun 10;16:17. doi: 10.1186/s12983-019-0320-2. eCollection 2019. Front Zool. 2019. PMID: 31198433 Free PMC article.
-
The evolutionary history and spectral tuning of vertebrate visual opsins.Dev Biol. 2023 Jan;493:40-66. doi: 10.1016/j.ydbio.2022.10.014. Epub 2022 Nov 9. Dev Biol. 2023. PMID: 36370769 Free PMC article. Review.
-
Diversity and Molecular Evolution of Nonvisual Opsin Genes across Environmental, Developmental, and Morphological Adaptations in Frogs.Mol Biol Evol. 2024 Jun 1;41(6):msae090. doi: 10.1093/molbev/msae090. Mol Biol Evol. 2024. PMID: 38736374 Free PMC article.
-
Visual adaptation of opsin genes to the aquatic environment in sea snakes.BMC Evol Biol. 2020 Nov 26;20(1):158. doi: 10.1186/s12862-020-01725-1. BMC Evol Biol. 2020. PMID: 33243140 Free PMC article.
-
Daily activity patterns influence retinal morphology, signatures of selection, and spectral tuning of opsin genes in colubrid snakes.BMC Evol Biol. 2017 Dec 11;17(1):249. doi: 10.1186/s12862-017-1110-0. BMC Evol Biol. 2017. PMID: 29228925 Free PMC article.
References
-
- Bowmaker JK, Astell S, Hunt DM, Mollon JD. Photosensitive and photostable pigments in the retinae of Old World monkeys. J Exp Biol. 1991;156:1–19. - PubMed
-
- Buck SL. Influence of rod signals on hue perception: evidence from successive scotopic contrast. Vision Res. 1997;37:1295–1301. - PubMed
-
- Cohen GB, Oprian DD, Robinson PR. Mechanism of activation and inactivation of opsin: role of Glu113 and Lys296. Biochemistry. 1992;31:12592–12601. - PubMed
-
- Conant R, Collins J. Boston: Houghton Mifflin; 1998. A field guide to reptiles and amphibians of eastern and central North America (Peterson field guide series)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
