The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse
- PMID: 19515924
- PMCID: PMC2727356
- DOI: 10.1523/JNEUROSCI.0514-09.2009
The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse
Abstract
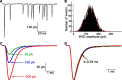
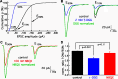
EPSCs at the synapses of sensory receptors and of some CNS neurons include large events thought to represent the synchronous release of the neurotransmitter contained in several synaptic vesicles by a process known as multiquantal release. However, determination of the unitary, quantal size underlying such putatively multiquantal events has proven difficult at hair cell synapses, hindering confirmation that large EPSCs are in fact multiquantal. Here, we address this issue by performing presynaptic membrane capacitance measurements together with paired recordings at the ribbon synapses of adult hair cells. These simultaneous presynaptic and postsynaptic assays of exocytosis, together with electron microscopic estimates of single vesicle capacitance, allow us to estimate a single vesicle EPSC charge of approximately -45 fC, a value in close agreement with the mean postsynaptic charge transfer of uniformly small EPSCs recorded during periods of presynaptic hyperpolarization. By thus establishing the magnitude of the fundamental quantal event at this peripheral sensory synapse, we provide evidence that the majority of spontaneous and evoked EPSCs are multiquantal. Furthermore, we show that the prevalence of uniquantal versus multiquantal events is Ca2+ dependent. Paired recordings also reveal a tight correlation between membrane capacitance increase and evoked EPSC charge, indicating that glutamate release during prolonged hair cell depolarization does not significantly saturate or desensitize postsynaptic AMPA receptors. We propose that the large EPSCs reflect the highly synchronized release of multiple vesicles at single presynaptic ribbon-type active zones through a compound or coordinated vesicle fusion mechanism.
Figures






References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Angleson JK, Betz WJ. Monitoring secretion in real time: capacitance, amperometry, fluorescence compared. Trends Neurosci. 1997;20:281–287. - PubMed
-
- Bekkers JM, Stevens CF. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. J Neurophysiol. 1996;75:1250–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
