Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity
- PMID: 19515926
- PMCID: PMC6665404
- DOI: 10.1523/JNEUROSCI.1336-09.2009
Role of amyloid-beta glycine 33 in oligomerization, toxicity, and neuronal plasticity
Abstract
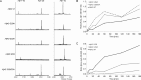
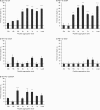
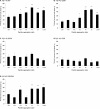
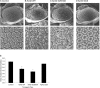
The aggregation of the amyloid-beta (Abeta) peptide plays a pivotal role in the pathogenesis of Alzheimer's disease, as soluble oligomers are intimately linked to neuronal toxicity and inhibition of hippocampal long-term potentiation (LTP). In the C-terminal region of Abeta there are three consecutive GxxxG dimerization motifs, which we could previously demonstrate to play a critical role in the generation of Abeta. Here, we show that glycine 33 (G33) of the central GxxxG interaction motif within the hydrophobic Abeta sequence is important for the aggregation dynamics of the peptide. Abeta peptides with alanine or isoleucine substitutions of G33 displayed an increased propensity to form higher oligomers, which we could attribute to conformational changes. Importantly, the oligomers of G33 variants were much less toxic than Abeta(42) wild type (WT), in vitro and in vivo. Also, whereas Abeta(42) WT is known to inhibit LTP, Abeta(42) G33 variants had lost the potential to inhibit LTP. Our findings reveal that conformational changes induced by G33 substitutions unlink toxicity and oligomerization of Abeta on the molecular level and suggest that G33 is the key amino acid in the toxic activity of Abeta. Thus, a specific toxic conformation of Abeta exists, which represents a promising target for therapeutic interventions.
Figures



References
-
- Basler K, Hafen E. Sevenless and Drosophila eye development: a tyrosine kinase controls cell fate. Trends Genet. 1988;4:74–79. - PubMed
-
- Beyermann M, Fechner K, Furkert J, Krause E, Bienert M. A single-point slight alteration set as a tool for structure-activity relationship studies of ovine corticotropin releasing factor. J Med Chem. 1996;39:3324–3330. - PubMed
-
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
