Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning
- PMID: 19515929
- PMCID: PMC6665418
- DOI: 10.1523/JNEUROSCI.0707-09.2009
Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning
Abstract
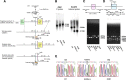
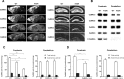
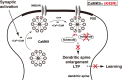
Ca2+/calmodulin-dependent protein kinase IIalpha (CaMKIIalpha) is an essential mediator of activity-dependent synaptic plasticity that possesses multiple protein functions. So far, the autophosphorylation site-mutant mice targeted at T286 and at T305/306 have demonstrated the importance of the autonomous activity and Ca2+/calmodulin-binding capacity of CaMKIIalpha, respectively, in the induction of long-term potentiation (LTP) and hippocampus-dependent learning. However, kinase activity of CaMKIIalpha, the most essential enzymatic function, has not been genetically dissected yet. Here, we generated a novel CaMKIIalpha knock-in mouse that completely lacks its kinase activity by introducing K42R mutation and examined the effects on hippocampal synaptic plasticity and behavioral learning. In homozygous CaMKIIalpha (K42R) mice, kinase activity was reduced to the same level as in CaMKIIalpha-null mice, whereas CaMKII protein expression was well preserved. Tetanic stimulation failed to induce not only LTP but also sustained dendritic spine enlargement, a structural basis for LTP, at the Schaffer collateral-CA1 synapse, whereas activity-dependent postsynaptic translocation of CaMKIIalpha was preserved. In addition, CaMKIIalpha (K42R) mice showed a severe impairment in inhibitory avoidance learning, a form of memory that is dependent on the hippocampus. These results demonstrate that kinase activity of CaMKIIalpha is a common critical gate controlling structural, functional, and behavioral expression of synaptic memory.
Figures




Similar articles
-
Developmental restoration of LTP deficits in heterozygous CaMKIIα KO mice.J Neurophysiol. 2016 Nov 1;116(5):2140-2151. doi: 10.1152/jn.00518.2016. Epub 2016 Aug 17. J Neurophysiol. 2016. PMID: 27535377 Free PMC article.
-
Differential Involvement of Kinase Activity of Ca2+/Calmodulin-Dependent Protein Kinase IIα in Hippocampus- and Amygdala-Dependent Memory Revealed by Kinase-Dead Knock-In Mouse.eNeuro. 2018 Aug 21;5(4):ENEURO.0133-18.2018. doi: 10.1523/ENEURO.0133-18.2018. eCollection 2018 Jul-Aug. eNeuro. 2018. PMID: 30225347 Free PMC article.
-
Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II.J Neurosci. 2007 Dec 19;27(51):14007-11. doi: 10.1523/JNEUROSCI.3587-07.2007. J Neurosci. 2007. PMID: 18094239 Free PMC article.
-
Interplay of enzymatic and structural functions of CaMKII in long-term potentiation.J Neurochem. 2016 Dec;139(6):959-972. doi: 10.1111/jnc.13672. Epub 2016 Jun 27. J Neurochem. 2016. PMID: 27207106 Review.
-
A role of Ca2+/calmodulin-dependent protein kinase II in the induction of long-term potentiation in hippocampal CA1 area.Neurosci Res. 1996 Jan;24(2):117-22. doi: 10.1016/0168-0102(95)00991-4. Neurosci Res. 1996. PMID: 8929917 Review.
Cited by
-
Effect of multimeric structure of CaMKII in the GluN2B-mediated modulation of kinetic parameters of ATP.PLoS One. 2012;7(9):e45064. doi: 10.1371/journal.pone.0045064. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028764 Free PMC article.
-
Studying CaMKII: Tools and standards.Cell Rep. 2024 Apr 23;43(4):113982. doi: 10.1016/j.celrep.2024.113982. Epub 2024 Mar 21. Cell Rep. 2024. PMID: 38517893 Free PMC article. Review.
-
Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses.J Cell Biol. 2012 Sep 17;198(6):1055-73. doi: 10.1083/jcb.201202058. Epub 2012 Sep 10. J Cell Biol. 2012. PMID: 22965911 Free PMC article.
-
CaMKII: a central molecular organizer of synaptic plasticity, learning and memory.Nat Rev Neurosci. 2022 Nov;23(11):666-682. doi: 10.1038/s41583-022-00624-2. Epub 2022 Sep 2. Nat Rev Neurosci. 2022. PMID: 36056211 Review.
-
Generation of Flag/DYKDDDDK Epitope Tag Knock-In Mice Using i-GONAD Enables Detection of Endogenous CaMKIIα and β Proteins.Int J Mol Sci. 2022 Oct 7;23(19):11915. doi: 10.3390/ijms231911915. Int J Mol Sci. 2022. PMID: 36233218 Free PMC article.
References
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. The α-Ca2+/calmodulin kinase II: a bidirectional modulator of presynaptic plasticity. Neuron. 1995;14:591–597. - PubMed
-
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
