Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia
- PMID: 19515981
- PMCID: PMC2724235
- DOI: 10.1152/ajpregu.90821.2008
Dorsal spinal cord stimulation obtunds the capacity of intrathoracic extracardiac neurons to transduce myocardial ischemia
Abstract
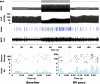
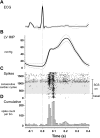
Populations of intrathoracic extracardiac neurons transduce myocardial ischemia, thereby contributing to sympathetic control of regional cardiac indices during such pathology. Our objective was to determine whether electrical neuromodulation using spinal cord stimulation (SCS) modulates such local reflex control. In 10 anesthetized canines, middle cervical ganglion neurons were identified that transduce the ventricular milieu. Their capacity to transduce a global (rapid ventricular pacing) vs. regional (transient regional ischemia) ventricular stress was tested before and during SCS (50 Hz, 0.2 ms duration at 90% MT) applied to the dorsal aspect of the T1 to T4 spinal cord. Rapid ventricular pacing and transient myocardial ischemia both activated cardiac-related middle cervical ganglion neurons. SCS obtunded their capacity to reflexly respond to the regional ventricular ischemia, but not rapid ventricular pacing. In conclusion, spinal cord inputs to the intrathoracic extracardiac nervous system obtund the latter's capacity to transduce regional ventricular ischemia, but not global cardiac stress. Given the substantial body of literature indicating the adverse consequences of excessive adrenergic neuronal excitation on cardiac function, these data delineate the intrathoracic extracardiac nervous system as a potential target for neuromodulation therapy in minimizing such effects.
Figures


References
-
- American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. - PubMed
-
- Andresen MC, Kunze DL, Mendelowitz D. Central nervous system regulation of the heart. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 187–219.
-
- Ardell JL Intrathoracic neuronal regulation of cardiac function. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 118–152.
-
- Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronic decentralized canine hearts. Am J Physiol Heart Circ Physiol 260: H713–H721, 1991. - PubMed
-
- Armour JA Synaptic transmission in chronically decentralized middle cervical and stellate ganglia of the dog. Can J Physiol Pharmacol 61: 1149–1155, 1983. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

