Rational approach to implementation of prostate cancer antigen 3 into clinical care
- PMID: 19517474
- PMCID: PMC2746943
- DOI: 10.1002/cncr.24447
Rational approach to implementation of prostate cancer antigen 3 into clinical care
Abstract
Background: Prostate cancer antigen 3 (PCA3) encodes a prostate-specific messenger ribonucleic acid (mRNA) that serves as the target for a novel urinary molecular assay for prostate cancer detection. The objective of the current study was to evaluate the ability of PCA3, added to measurements of serum prostate-specific antigen (PSA), to predict cancer detection by extended template biopsy.
Methods: Between September 2006 and December 2007, whole urine samples were collected after attentive digital rectal examinations from 187 men before they underwent ultrasound-guided, 12-core prostate biopsy in a urology outpatient clinic. Urine PCA3/PSA mRNA ratio scores were measured within 1 month, and serum PSA was measured within 6 months prior to biopsy. Those measurements were related to cancer-positive biopsies.
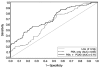
Results: Overall, 87 of 187 biopsies (46.5%) were positive for cancer. The sensitivity and specificity of a PCA3 score > or =35 for positive biopsy were 52.9% and 80%, respectively, and the positive and negative predictive values were 69.7% and 66.1%, respectively. By using receiver operating characteristic curve analysis, PSA alone resulted in an area under the curve (AUC) of 0.63 for prostate cancer detection; whereas a combined PSA and PCA3 score resulted in an AUC of 0.71. The likelihood of prostate cancer detection rose with increasing PCA3 score ranges (P > .0001), providing possible PCA3 score parameters for stratification into groups at low risk, moderate risk, high risk, and very high risk for a positive biopsy.
Conclusions: Adding PCA3 to serum PSA improved prostate cancer prediction. The use of PCA3 in a clinical setting may help to stratify patients according to their risk for biopsy and cancer detection, although a large-scale validation study will be needed to address assay standardization, optimal cutoff values, and appropriate patient populations.
Figures

References
-
- Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17(2):159–63. - PubMed
-
- Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270(8):948–54. - PubMed
-
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–46. - PubMed
-
- Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–7. see comment. - PubMed
-
- Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

