Putative heterotopic ossification progenitor cells derived from traumatized muscle
- PMID: 19517576
- PMCID: PMC3014572
- DOI: 10.1002/jor.20924
Putative heterotopic ossification progenitor cells derived from traumatized muscle
Abstract
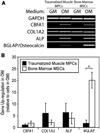
Heterotopic ossification (HO) is a frequent complication following combat-related trauma, but the pathogenesis of traumatic HO is poorly understood. Building on our recent identification of mesenchymal progenitor cells (MPCs) in traumatically injured muscle, the goal of this study was to evaluate the osteogenic potential of the MPCs in order to assess the role of these cells in HO formation. Compared to bone marrow-derived mesenchymal stem cells (MSCs), a well-characterized population of osteoprogenitor cells, the MPCs exhibited several significant differences during osteogenic differentiation and in the expression of genes related to osteogenesis. Upon osteogenic induction, MPCs showed increased alkaline phosphatase activity, production of a mineralized matrix, and up-regulated expression of the osteoblast-associated genes CBFA1 and alkaline phosphatase. However, MPCs did not appear to reach terminal differentiation as the expression of osteocalcin was not substantially up-regulated. With the exception of a few genes, the osteogenic gene expression profile of traumatized muscle-derived MPCs was comparable to that of the MSCs after osteogenic induction. These findings indicate that traumatized muscle-derived MPCs have the potential to function as osteoprogenitor cells when exposed to the appropriate biochemical environment and are the putative osteoprogenitor cells that initiate ectopic bone formation in HO.
Figures




References
-
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. - PubMed
-
- Pape HC, Marsh S, Morley JR, et al. Current concepts in the development of heterotopic ossification. J Bone Joint Surg Br. 2004;86:783–787. - PubMed
-
- Kaplan FS, Glaser DL, Hebela N, et al. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12:116–125. - PubMed
-
- Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

