PI(3,4,5)P3 potentiates phospholipase C-beta activity
- PMID: 19519170
- PMCID: PMC2830898
- DOI: 10.1080/10799890902729449
PI(3,4,5)P3 potentiates phospholipase C-beta activity
Abstract
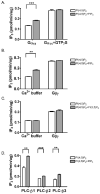
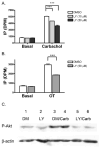
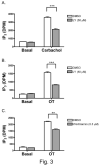
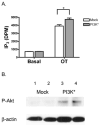
Phospholipase C-beta (PLC-beta) isozymes are key effectors in G protein-coupled signaling pathways. Previously, we showed that PLC-beta1 and PLC-beta3 bound immobilized PIP(3). In this study, PIP(3) was found to potentiate Ca(2+)-stimulated PLC-beta activities using an in vitro reconstitution assay. LY294002, a specific PI 3-kinase inhibitor, significantly inhibited 10 min of agonist-stimulated total IP accumulation. Both LY294002 and wortmannin inhibited 90 sec of agonist-stimulated IP(3) accumulation in intact cells. Moreover, transfected p110CAAX, a constitutively activated PI 3-kinase catalytic subunit, increased 90 sec of oxytocin-stimulated IP(3) accumulation. Receptor-ligand binding assays indicated that LY294002 did not affect G protein-coupled receptors directly, suggesting a physiological role for PIP(3) in directly potentiating PLC-beta activity. When coexpressed with p110CAAX, fluorescence-tagged PLC-beta3 was increasingly localized to the plasma membrane. Additional observations suggest that the PH domain of PLC-beta is not important for p110CAAX-induced membrane association.
Figures


Similar articles
-
Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-gamma2 is an early key event in FcgammaRIIA-mediated activation of human platelets.J Biol Chem. 1998 Sep 18;273(38):24314-21. doi: 10.1074/jbc.273.38.24314. J Biol Chem. 1998. PMID: 9733717
-
Phospholipase C-gamma mediates the hydrolysis of phosphatidylinositol, but not of phosphatidylinositol 4,5-bisphoshate, in carbamylcholine-stimulated islets of langerhans.J Biol Chem. 2001 Jun 1;276(22):19072-7. doi: 10.1074/jbc.M101406200. Epub 2001 Mar 27. J Biol Chem. 2001. PMID: 11274217
-
Thrombin stimulates wortmannin-inhibitable phosphoinositide 3-kinase and membrane blebbing in CHRF-288 cells.Biochem J. 1996 Mar 15;314 ( Pt 3)(Pt 3):805-10. doi: 10.1042/bj3140805. Biochem J. 1996. PMID: 8615773 Free PMC article.
-
Subtype-specific roles of phospholipase C-β via differential interactions with PDZ domain proteins.Adv Enzyme Regul. 2011;51(1):138-51. doi: 10.1016/j.advenzreg.2010.10.004. Epub 2010 Oct 28. Adv Enzyme Regul. 2011. PMID: 21035486 Review.
-
Nuclear inositides: PI-PLC signaling in cell growth, differentiation and pathology.Adv Enzyme Regul. 2009;49(1):2-10. doi: 10.1016/j.advenzreg.2008.12.001. Epub 2008 Dec 31. Adv Enzyme Regul. 2009. PMID: 19159640 Review. No abstract available.
Cited by
-
Endocytosis and the internalization of pathogenic organisms: focus on phosphoinositides.F1000Res. 2020 May 15;9:F1000 Faculty Rev-368. doi: 10.12688/f1000research.22393.1. eCollection 2020. F1000Res. 2020. PMID: 32494357 Free PMC article. Review.
-
Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes.Mol Biol Cell. 2013 Jun;24(11):1700-12, S1-7. doi: 10.1091/mbc.E12-11-0789. Epub 2013 Apr 10. Mol Biol Cell. 2013. PMID: 23576545 Free PMC article.
-
Multiple implications of 3-phosphoinositide-dependent protein kinase 1 in human cancer.World J Biol Chem. 2010 Aug 26;1(8):239-47. doi: 10.4331/wjbc.v1.i8.239. World J Biol Chem. 2010. PMID: 21537480 Free PMC article.
References
-
- Harden TK, Sondek J. Annu Rev Pharmacol Toxicol. 2006;46:355–379. - PubMed
-
- Wang T, Pentyala S, Rebecchi MJ, Scarlata S. Biochemistry. 1999;38:1517–1524. - PubMed
-
- Jezyk MR, Snyder JT, Gershberg S, Worthylake DK, Harden TK, Sondek J. Nat Struct Mol Biol. 2006;13(12):1135–1140. - PubMed
-
- Kim CG, Park D, Rhee SG. J Biol Chem. 1996;271:21187–21192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
