Adiponectin identified as an agonist for PAQR3/RKTG using a yeast-based assay system
- PMID: 19519172
- PMCID: PMC2792888
- DOI: 10.1080/10799890902729456
Adiponectin identified as an agonist for PAQR3/RKTG using a yeast-based assay system
Abstract
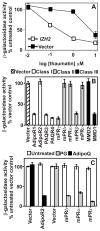
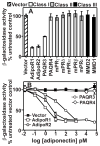
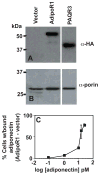
The PAQR family of proteins comprises an intriguing group of newly discovered receptors. Although the agonist is known for 5 of the 11 human PAQRs, most are considered "orphan" receptors. We developed a yeast-based assay system for PAQR receptor activity that can be used to identify agonists for PAQRs of unknown function. Using this system, we found that the proteinaceous hormone adiponectin functions as an agonist of PAQR3, a previously uncharacterized member of this family. This is not surprising given that PAQR3 is most closely related to PAQR1 (AdipoR1) and PAQR2 (AdipoR2), which also sense adiponectin. The identification of adiponectin as an agonist for PAQR3 is of considerable clinical relevance because adiponectin suppresses the proliferation of tumor cells and it has been reported that PAQR3 suppresses tumorigenesis. Thus, the interaction between PAQR3 and adiponectin may help explain the antiproliferative properties of adiponectin.
Figures

Similar articles
-
A Saccharomyces cerevisiae assay system to investigate ligand/AdipoR1 interactions that lead to cellular signaling.PLoS One. 2013 Jun 7;8(6):e65454. doi: 10.1371/journal.pone.0065454. Print 2013. PLoS One. 2013. PMID: 23762377 Free PMC article.
-
[Expression and Clinical Significance of Progesterone and Adiponectin Receptor Family Member 3 in Lung Cancer].Zhongguo Fei Ai Za Zhi. 2017 Apr 20;20(4):259-263. doi: 10.3779/j.issn.1009-3419.2017.04.06. Zhongguo Fei Ai Za Zhi. 2017. PMID: 28442015 Free PMC article. Chinese.
-
PAQR3 inhibits the proliferation, migration and invasion in human glioma cells.Biomed Pharmacother. 2017 Aug;92:24-32. doi: 10.1016/j.biopha.2017.05.046. Epub 2017 May 18. Biomed Pharmacother. 2017. PMID: 28528182
-
PAQR3: a novel tumor suppressor gene.Am J Cancer Res. 2015 Aug 15;5(9):2562-8. eCollection 2015. Am J Cancer Res. 2015. PMID: 26609468 Free PMC article. Review.
-
Paradigm shift: the primary function of the "Adiponectin Receptors" is to regulate cell membrane composition.Lipids Health Dis. 2021 Apr 30;20(1):43. doi: 10.1186/s12944-021-01468-y. Lipids Health Dis. 2021. PMID: 33931104 Free PMC article. Review.
Cited by
-
Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation.Circ Res. 2014 Feb 28;114(5):792-805. doi: 10.1161/CIRCRESAHA.114.302439. Epub 2014 Jan 7. Circ Res. 2014. PMID: 24397980 Free PMC article.
-
PAQR4 regulates adipocyte function and systemic metabolic health by mediating ceramide levels.Nat Metab. 2024 Jul;6(7):1347-1366. doi: 10.1038/s42255-024-01078-9. Epub 2024 Jul 3. Nat Metab. 2024. PMID: 38961186 Free PMC article.
-
A Saccharomyces cerevisiae assay system to investigate ligand/AdipoR1 interactions that lead to cellular signaling.PLoS One. 2013 Jun 7;8(6):e65454. doi: 10.1371/journal.pone.0065454. Print 2013. PLoS One. 2013. PMID: 23762377 Free PMC article.
-
Phylogenetic and preliminary phenotypic analysis of yeast PAQR receptors: potential antifungal targets.J Mol Evol. 2011 Oct;73(3-4):134-52. doi: 10.1007/s00239-011-9462-3. Epub 2011 Oct 19. J Mol Evol. 2011. PMID: 22009226 Free PMC article.
-
Modulation of gene expression by 3-iodothyronamine: genetic evidence for a lipolytic pattern.PLoS One. 2014 Nov 7;9(11):e106923. doi: 10.1371/journal.pone.0106923. eCollection 2014. PLoS One. 2014. PMID: 25379707 Free PMC article.
References
-
- Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: A novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61(3):372–380. - PubMed
-
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–769. - PubMed
-
- Brauer AU, Nitsch R, Savaskan NE. Identification of macrophage/microglia activationfactor (MAF) associated with late endosomes/lysosomes in microglial cells. FEBS Lett. 2004;563(1–3):41–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
