Aripiprazole in pervasive developmental disorder not otherwise specified and Asperger's disorder: a 14-week, prospective, open-label study
- PMID: 19519261
- PMCID: PMC2872206
- DOI: 10.1089/cap.2008.093
Aripiprazole in pervasive developmental disorder not otherwise specified and Asperger's disorder: a 14-week, prospective, open-label study
Abstract
Objective: The aim of this study was to determine the effectiveness and tolerability of aripiprazole for irritability in pervasive developmental disorder not otherwise specified (PDD-NOS) and Asperger's disorder.
Method: This is a 14-week, prospective, open-label investigation of aripiprazole in 25 children and adolescents diagnosed with PDD-NOS or Asperger's disorder. Primary outcome measures included the Clinical Global Impressions-Improvement (CGI-I) scale and the Irritability subscale of the Aberrant Behavior Checklist (ABC-I).
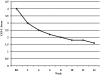
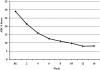
Results: Twenty-five subjects, ages 5-17 years (mean 8.6 years) received a mean final aripiprazole dosage of 7.8 mg/day (range 2.5-15 mg/day). Full-scale intelligence quotient (IQ) scores ranged from 48 to 122 (mean 84). Twenty-two (88%) of 25 subjects were responders in regard to interfering symptoms of irritability, including aggression, self-injury, and tantrums, with a final CGI-I of 1 or 2 (very much or much improved) and a 25% or greater improvement on the ABC-I. The final mean CGI-I was 1.6 (p <or= 0.0001). ABC-I scores ranged from 18 to 43 (mean 29) at baseline, whereas scores at week 14 ranged from 0 to 27 (mean 8.1) (p <or= 0.001). Aripiprazole was well tolerated. Mild extrapyramidal symptoms (EPS) were reported in 9 subjects. Age- and sex-normed body mass index (BMI) increased from a mean value of 20.3 at baseline to 21.1 at end point (p <or= 0.04). Prolactin significantly decreased from a mean value of 9.3 at baseline to 2.9 at end point (p <or= 0.0001). No subject exited the study due to a drug-related adverse event.
Conclusions: These preliminary data suggest that aripiprazole may be effective and well tolerated for severe irritability in pediatric patients with PDD-NOS or Asperger's disorder. Larger-scale placebo-controlled studies are needed to elucidate the efficacy and tolerability of aripiprazole in this understudied population.
Figures
References
-
- Aman MG. Singh NN. East Aurora (New York): Slosson Educational Publications; 1994. Supplement to Aberrant Behavior Checklist Manual.
-
- Aman MG. Singh NN. Stewart AW. Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–491. - PubMed
-
- Aman MG. Arnold LE. McDougle CJ. Vitiello B. Scahill L. Davies M. McCracken JT. Tierney E. Nash PL. Posey DJ. Chuang S. Martin A. Shah B. Gonzalez NM. Swiezy NB. Ritz L. Koenig K. McGough J. Ghuman JK. Lindsay RL. Acute and long term safety and tolerability of risperidone in children with autism. J Child Adolesc Psychopharmacol. 2005;25:565–569. - PubMed
-
- American Psychiatric Association. Text Revision. 4th. Washington (DC): American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders.
-
- Anderson GM. Scahill L. McCracken JT. McDougle CJ. Aman MG. Tierney E. Arnold LE. Martin A. Katsovich L. Posey DJ. Shah B. Vitiello B. Effects of short- and long-term risperidone treatment on prolactin levels in children and adolescents with autism. Biol Psychiatry. 2007;61:545–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources