1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets
- PMID: 19519378
- PMCID: PMC3326067
- DOI: 10.2174/092986709788682218
1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets
Abstract
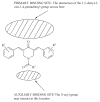
A number of organic molecules which contain the 1,5-diaryl-3-oxo-1,4-pentadienyl group, referred to hereafter as the dienone moiety, have antineoplastic properties. Emphasis is made on the attachment of this structural moiety to several molecular scaffolds, namely piperidines, N-acylpiperidines, cycloalkanes and 3,4-dihydro-1H-napthalenes. Many of these compounds are potent cytotoxins having micromolar and nanomolar IC(50) values towards a wide range of neoplastic and transformed cells. On occasions, greater toxicity towards neoplasms than normal cells has been demonstrated. A number of these compounds have in vivo anticancer properties and in general excellent tolerability in rodents is demonstrated. The way in which a number of physicochemical properties such as redox potentials, torsion angles, atomic charges and logP values govern cytotoxic potencies are presented. The importance of the shapes of different compounds as determined by molecular modeling in contributing to antineoplastic properties is outlined. Arguments are presented in favour of designing antineoplastics which have multiple sites of action in contrast to those bioactive molecules which have only one molecular target. A number of compounds which possess the dienone group have different modes of action some of which are chronicled in this review, such as inducing apoptosis, affecting respiration in mitochondria, inhibiting macromolecular biosynthesis and both inhibiting and stimulating certain enzymes. Other important properties of these compounds are discussed including their anti-angiogenic, MDR-revertant and antioxidant properties. It is hoped that this eulogy of the importance of the dienone group will encourage researchers to consider incorporating this structural unit into candidate cytotoxins in the future.
Figures





References
-
- Dimmock JR, Wong MLC. Bioactivities and potential uses in drug design of acyclic α, β-unsaturated ketones. Can J Pharm Sci. 1976;11:35–53.
-
- Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Antileukemic evaluation of some Mannich bases derived from 2-arylidene-1,3-diketones. Eur J Med Chem. 1983;18:248–254.
-
- Mutus B, Wagner JD, Talpas CJ, Dimmock JR, Phillips OA, Reid RS. 1-p-Chlorophenyl-4.4-dimethyl-5-diethylamino-1-penten-3-one hydrobromide, a sulfhydryl-specific compound which reacts irreversibly with protein thiols but reversibly with small molecular weight thiols. Anal Biochem. 1989;177:237–243. - PubMed
-
- Benvenuto JA, Connor TH, Monteith DK, Laidlaw JA, Adams SC, Matney TS, Theiss JC. Degradation and inactivation of antitumor drugs. J Pharm Sci. 1993;82:988–991. - PubMed
-
- Okey AB, Harper PA. In: Principles of Medical Pharmacology. 7. Kalant H, Grant DM, Mitchell J, editors. Elsevier; Toronto, Canada: 2007. p. 902.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

