Scientific and clinical challenges in sepsis
- PMID: 19519432
- PMCID: PMC3098530
- DOI: 10.2174/138161209788453248
Scientific and clinical challenges in sepsis
Abstract
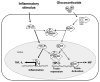
Advances in intensive care and antibiotics have prevented the spread of some infections, though sepsis mortality rates remain high. With failure of over thirty clinical trials, sepsis remains a scientific and clinical challenge in modern medicine. Sepsis is defined by the clinical signs of a systemic inflammatory response to infection. "Severe sepsis" is when these symptoms are associated with multiple organ dysfunction. These definitions of sepsis may be too broad and common to heterogeneous groups of patients who do not necessarily have the same disorder. This consideration has become especially evident in the clinical trials that have failed to obtain consistent results in similar studies of patients diagnosed with severe sepsis. In these trials, patients with infections caused by different microorganisms, and affecting different organs, have been combined under the general diagnosis of severe sepsis. The situation is analogous to attempting a clinical trial based on the general definition of cancer, combining all patients with tumor independent of the type of malignancy. In this consideration, it would not be very surprising that activated protein C, the only treatment in sepsis approved by the Food and Drug Administration, is projected for use in only a small subset of patients with severe sepsis. This article reviews novel inflammatory molecular aspects and the experimental anti-inflammatory strategies for sepsis, as they may represent particular pathological processes in specific subsets of patients.
Figures


References
-
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–84. - PubMed
-
- Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. - PubMed
-
- Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. - PubMed
-
- Deitch EA, Dayal SD. Intensive care unit management of the trauma patient. Crit Care Med. 2006;34:2294–301. - PubMed
-
- Mantell LL, Parrish WR, Ulloa L. Hmgb-1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

