Interaction of catechol and non-catechol substrates with externally or internally facing dopamine transporters
- PMID: 19519772
- PMCID: PMC2696066
- DOI: 10.1111/j.1471-4159.2009.06034.x
Interaction of catechol and non-catechol substrates with externally or internally facing dopamine transporters
Abstract
Our previous work suggested that collapsing the Na(+) gradient and membrane potential converts the dopamine (DA) transporter (DAT) to an inward-facing conformation with a different substrate binding profile. Here, DAT expressing human embryonic kidney 293 cells were permeabilized with digitonin, disrupting ion/voltage gradients and allowing passage of DAT substrates. The potency of p-tyramine and other non-catechols (d-amphetamine, beta-phenethylamine, MPP(+)) in inhibiting cocaine analog binding to DAT in digitonin-treated cells was markedly weakened to a level similar to that observed in cell-free membranes. In contrast, the potency of DA and another catechol, norepinephrine, was not significantly changed by the same treatment, whereas epinephrine showed only a modest reduction. These findings suggest that catechol substrates interact symmetrically with both sides of DAT and non-catechol substrates, favoring binding to outward-facing transporter. In the cocaine analog binding assay, the mutant W84L displayed enhanced intrinsic binding affinity for substrates in interacting with both outward- and inward-facing states; D313N showed wild-type-like symmetric binding; but D267L and E428Q showed an apparent improvement in the permeation pathway from the external face towards the substrate site. Thus, the structure of both substrate and transporter play a role in the sidedness and mode of interaction between them.
Figures



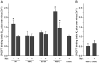


Similar articles
-
Bivalent phenethylamines as novel dopamine transporter inhibitors: evidence for multiple substrate-binding sites in a single transporter.J Neurochem. 2010 Mar;112(6):1605-18. doi: 10.1111/j.1471-4159.2010.06583.x. Epub 2010 Jan 12. J Neurochem. 2010. PMID: 20067583 Free PMC article.
-
Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells.J Neurochem. 2007 Apr;101(2):377-88. doi: 10.1111/j.1471-4159.2006.04384.x. Epub 2007 Jan 22. J Neurochem. 2007. PMID: 17250655
-
Targeting of dopamine transporter to filopodia requires an outward-facing conformation of the transporter.Sci Rep. 2017 Jul 14;7(1):5399. doi: 10.1038/s41598-017-05637-x. Sci Rep. 2017. PMID: 28710426 Free PMC article.
-
Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter.Drug Alcohol Depend. 2015 Feb 1;147:1-19. doi: 10.1016/j.drugalcdep.2014.12.005. Epub 2014 Dec 18. Drug Alcohol Depend. 2015. PMID: 25548026 Free PMC article. Review.
-
A comprehensive atlas of the topography of functional groups of the dopamine transporter.Synapse. 2005 Nov;58(2):72-94. doi: 10.1002/syn.20183. Synapse. 2005. PMID: 16088952 Review.
Cited by
-
Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration.Biol Psychiatry. 2011 Feb 1;69(3):201-7. doi: 10.1016/j.biopsych.2010.06.026. Biol Psychiatry. 2011. PMID: 20801429 Free PMC article.
-
Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling.Psychopharmacology (Berl). 2017 Sep;234(18):2761-2776. doi: 10.1007/s00213-017-4673-y. Epub 2017 Jun 30. Psychopharmacology (Berl). 2017. PMID: 28667509 Free PMC article.
-
Dopamine transporter oligomerization: impact of combining protomers with differential cocaine analog binding affinities.J Neurochem. 2015 Apr;133(2):167-73. doi: 10.1111/jnc.13025. Epub 2015 Jan 26. J Neurochem. 2015. PMID: 25580950 Free PMC article.
-
In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat.J Pharmacol Exp Ther. 2011 Apr;337(1):218-25. doi: 10.1124/jpet.110.176271. Epub 2011 Jan 12. J Pharmacol Exp Ther. 2011. PMID: 21228061 Free PMC article.
-
Dopamine transporter function fluctuates across sleep/wake state: potential impact for addiction.Neuropsychopharmacology. 2021 Mar;46(4):699-708. doi: 10.1038/s41386-020-00879-2. Epub 2020 Oct 8. Neuropsychopharmacology. 2021. PMID: 33032296 Free PMC article.
References
-
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. - PubMed
-
- Amejdki-Chab N, Costentin J, Bonnet JJ. Kinetic analysis of the chloride dependence of the neuronal uptake of dopamine and effect of anions on the ability of substrates to compete with the binding of the dopamine uptake inhibitor GBR 12783. J. Neurochem. 1992;58:793–800. - PubMed
-
- Appell M, Berfield JL, Wang LC, Dunn WJ, III, Chen N, Reith ME. Structure-activity relationships for substrate recognition by the human dopamine transporter. Biochem. Pharmacol. 2004;67:293–302. - PubMed
-
- Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicol. Appl. Pharmacol. 2005;204:355–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

