Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure
- PMID: 19519782
- PMCID: PMC2696043
- DOI: 10.1111/j.1471-4159.2009.06055.x
Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure
Abstract
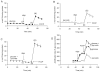
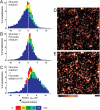
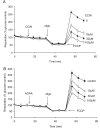
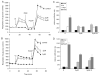
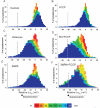
Pre-synaptic nerve terminals (synaptosomes) require ATP for neurotransmitter exocytosis and recovery and for ionic homeostasis, and are consequently abundantly furnished with mitochondria. Pre-synaptic mitochondrial dysfunction is implicated in a variety of neurodegenerative disorders, although there is no precise definition of the term 'dysfunction'. In this study, we test the hypothesis that partial restriction of electron transport through Complexes I and II in synaptosomes to mimic possible defects associated with Parkinson's and Huntington's diseases respectively, sensitizes individual terminals to mitochondrial depolarization under conditions of enhanced proton current utilization, even though these stresses are within the respiratory capacity of the synaptosomes when averaged over the entire population. We combine two novel techniques, firstly using a modification of a plate-based respiration and glycolysis assay that requires only microgram quantities of synaptosomal protein, and secondly developing an improved method for fluorescent imaging and statistical analysis of single synaptosomes. Conditions are defined for optimal substrate supply to the in situ mitochondria within mouse cerebrocortical synaptosomes, and the energetic demands of ion cycling and action-potential firing at the plasma membrane are additionally determined.
Figures


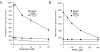
References
-
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58:495–505. - PubMed
-
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol. Dis. 2006;22:404–420. - PubMed
-
- Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005;33:897–904. - PubMed
-
- Browne SE, Beal MF. The energetics of Huntington's disease. Neurochem. Res. 2004;29:531–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

