Synthetic lethal RNAi screening identifies sensitizing targets for gemcitabine therapy in pancreatic cancer
- PMID: 19519883
- PMCID: PMC2702280
- DOI: 10.1186/1479-5876-7-43
Synthetic lethal RNAi screening identifies sensitizing targets for gemcitabine therapy in pancreatic cancer
Abstract
Background: Pancreatic cancer retains a poor prognosis among the gastrointestinal cancers. It affects 230,000 individuals worldwide, has a very high mortality rate, and remains one of the most challenging malignancies to treat successfully. Treatment with gemcitabine, the most widely used chemotherapeutic against pancreatic cancer, is not curative and resistance may occur. Combinations of gemcitabine with other chemotherapeutic drugs or biological agents have resulted in limited improvement.
Methods: In order to improve gemcitabine response in pancreatic cancer cells, we utilized a synthetic lethal RNAi screen targeting 572 known kinases to identify genes that when silenced would sensitize pancreatic cancer cells to gemcitabine.
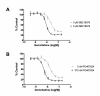
Results: Results from the RNAi screens identified several genes that, when silenced, potentiated the growth inhibitory effects of gemcitabine in pancreatic cancer cells. The greatest potentiation was shown by siRNA targeting checkpoint kinase 1 (CHK1). Validation of the screening results was performed in MIA PaCa-2 and BxPC3 pancreatic cancer cells by examining the dose response of gemcitabine treatment in the presence of either CHK1 or CHK2 siRNA. These results showed a three to ten-fold decrease in the EC50 for CHK1 siRNA-treated cells versus control siRNA-treated cells while treatment with CHK2 siRNA resulted in no change compared to controls. CHK1 was further targeted with specific small molecule inhibitors SB 218078 and PD 407824 in combination with gemcitabine. Results showed that treatment of MIA PaCa-2 cells with either of the CHK1 inhibitors SB 218078 or PD 407824 led to sensitization of the pancreatic cancer cells to gemcitabine.
Conclusion: These findings demonstrate the effectiveness of synthetic lethal RNAi screening as a tool for identifying sensitizing targets to chemotherapeutic agents. These results also indicate that CHK1 could serve as a putative therapeutic target for sensitizing pancreatic cancer cells to gemcitabine.
Figures



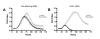
Similar articles
-
Context dependence of checkpoint kinase 1 as a therapeutic target for pancreatic cancers deficient in the BRCA2 tumor suppressor.Mol Cancer Ther. 2011 Apr;10(4):670-8. doi: 10.1158/1535-7163.MCT-10-0781. Epub 2011 Feb 2. Mol Cancer Ther. 2011. PMID: 21289082 Free PMC article.
-
Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells.Mol Cancer Ther. 2009 Jan;8(1):45-54. doi: 10.1158/1535-7163.MCT-08-0662. Mol Cancer Ther. 2009. PMID: 19139112 Free PMC article.
-
RNAi screening of the kinome identifies modulators of cisplatin response in ovarian cancer cells.Gynecol Oncol. 2010 Sep;118(3):220-7. doi: 10.1016/j.ygyno.2010.05.006. Gynecol Oncol. 2010. PMID: 20722101
-
HuR's role in gemcitabine efficacy: an exception or opportunity?Wiley Interdiscip Rev RNA. 2011 May-Jun;2(3):435-44. doi: 10.1002/wrna.62. Epub 2010 Nov 30. Wiley Interdiscip Rev RNA. 2011. PMID: 21957028 Review.
-
Natural Anti-Cancer Agents: Implications in Gemcitabine-Resistant Pancreatic Cancer Treatment.Mini Rev Med Chem. 2017;17(11):920-927. doi: 10.2174/1389557517666170315124438. Mini Rev Med Chem. 2017. PMID: 28302042 Review.
Cited by
-
Chemotherapy and signaling: How can targeted therapies supercharge cytotoxic agents?Cancer Biol Ther. 2010 Nov 1;10(9):839-53. doi: 10.4161/cbt.10.9.13738. Epub 2010 Nov 1. Cancer Biol Ther. 2010. PMID: 20935499 Free PMC article. Review.
-
RPS3 regulates melanoma cell growth and apoptosis by targeting Cyto C/Ca2+/MICU1 dependent mitochondrial signaling.Oncotarget. 2015 Oct 6;6(30):29614-25. doi: 10.18632/oncotarget.4868. Oncotarget. 2015. PMID: 26336993 Free PMC article.
-
A novel siRNA-gemcitabine construct as a potential therapeutic for treatment of pancreatic cancer.NAR Cancer. 2020 Aug 11;2(3):zcaa016. doi: 10.1093/narcan/zcaa016. eCollection 2020 Sep. NAR Cancer. 2020. PMID: 34316688 Free PMC article.
-
The kinome 'at large' in cancer.Nat Rev Cancer. 2016 Feb;16(2):83-98. doi: 10.1038/nrc.2015.18. Nat Rev Cancer. 2016. PMID: 26822576 Review.
-
Inactivation of mirk/dyrk1b kinase targets quiescent pancreatic cancer cells.Mol Cancer Ther. 2011 Nov;10(11):2104-14. doi: 10.1158/1535-7163.MCT-11-0498. Epub 2011 Aug 30. Mol Cancer Ther. 2011. PMID: 21878655 Free PMC article.
References
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2',2'-difluoro-2'-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. - PubMed
-
- Bepler G, Sommers KE, Cantor A, Li X, Sharma A, Williams C, Chiappori A, Haura E, Antonia S, Tanvetyanon T, Simon G, Obasaju C, Robinson LA. Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer. J Thorac Oncol. 2008;3:1112–1118. doi: 10.1097/JTO.0b013e3181874936. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

