Issues in applying multi-arm multi-stage methodology to a clinical trial in prostate cancer: the MRC STAMPEDE trial
- PMID: 19519885
- PMCID: PMC2704188
- DOI: 10.1186/1745-6215-10-39
Issues in applying multi-arm multi-stage methodology to a clinical trial in prostate cancer: the MRC STAMPEDE trial
Abstract
Background: The multi-arm multi-stage (MAMS) trial is a new paradigm for conducting randomised controlled trials that allows the simultaneous assessment of a number of research treatments against a single control arm. MAMS trials provide earlier answers and are potentially more cost-effective than a series of traditionally designed trials. Prostate cancer is the most common tumour in men and there is a need to improve outcomes for men with hormone-sensitive, advanced disease as quickly as possible. The MAMS design will potentially facilitate evaluation and testing of new therapies in this and other diseases.
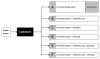
Methods: STAMPEDE is an open-label, 5-stage, 6-arm randomised controlled trial using MAMS methodology for men with prostate cancer. It is the first trial of this design to use multiple arms and stages synchronously.
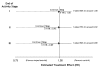
Results: The practical and statistical issues faced by STAMPEDE in implementing MAMS methodology are discussed and contrasted with those for traditional trials. These issues include the choice of intermediate and final outcome measures, sample size calculations and the impact of varying the assumptions, the process for moving between trial stages, stopping accrual to each trial arm and overall, and issues around perceived trial complexity.
Conclusion: It is possible to use the MAMS design to initiate and undertake large scale cancer trials. The results from STAMPEDE will not be known for some years but the lessons learned from running a MAMS trial are shared in the hope that other researchers will use this exciting and efficient method to perform further randomised controlled trials.
Trial registration: ISRCTN78818544, NCT00268476.
Figures



References
-
- James N, Mason M, Sydes M, Sanders K, Dearnaley D, Anderson J, et al. Successful recruitment to the feasibility stage of STAMPEDE: A multi-arm, multi-stage phase II/III trial in high risk prostate cancer (ISRCTN78818544) Proc ASCO Prostate Cancer Symposium. 2007.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

