Estimating the number needed to treat from continuous outcomes in randomised controlled trials: methodological challenges and worked example using data from the UK Back Pain Exercise and Manipulation (BEAM) trial
- PMID: 19519911
- PMCID: PMC2702335
- DOI: 10.1186/1471-2288-9-35
Estimating the number needed to treat from continuous outcomes in randomised controlled trials: methodological challenges and worked example using data from the UK Back Pain Exercise and Manipulation (BEAM) trial
Abstract
Background: Reporting numbers needed to treat (NNT) improves interpretability of trial results. It is unusual that continuous outcomes are converted to numbers of individual responders to treatment (i.e., those who reach a particular threshold of change); and deteriorations prevented are only rarely considered. We consider how numbers needed to treat can be derived from continuous outcomes; illustrated with a worked example showing the methods and challenges.
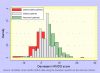
Methods: We used data from the UK BEAM trial (n = 1, 334) of physical treatments for back pain; originally reported as showing, at best, small to moderate benefits. Participants were randomised to receive 'best care' in general practice, the comparator treatment, or one of three manual and/or exercise treatments: 'best care' plus manipulation, exercise, or manipulation followed by exercise. We used established consensus thresholds for improvement in Roland-Morris disability questionnaire scores at three and twelve months to derive NNTs for improvements and for benefits (improvements gained+deteriorations prevented).
Results: At three months, NNT estimates ranged from 5.1 (95% CI 3.4 to 10.7) to 9.0 (5.0 to 45.5) for exercise, 5.0 (3.4 to 9.8) to 5.4 (3.8 to 9.9) for manipulation, and 3.3 (2.5 to 4.9) to 4.8 (3.5 to 7.8) for manipulation followed by exercise. Corresponding between-group mean differences in the Roland-Morris disability questionnaire were 1.6 (0.8 to 2.3), 1.4 (0.6 to 2.1), and 1.9 (1.2 to 2.6) points.
Conclusion: In contrast to small mean differences originally reported, NNTs were small and could be attractive to clinicians, patients, and purchasers. NNTs can aid the interpretation of results of trials using continuous outcomes. Where possible, these should be reported alongside mean differences. Challenges remain in calculating NNTs for some continuous outcomes.
Trial registration: UK BEAM trial registration: ISRCTN32683578.
Figures
References
-
- Rose G. The strategy of preventive medicine. Oxford, United Kingdom: Oxford University Press; 1992. Individuals and populations; pp. 12,53–63,74.
-
- Cohen J. Statistical power analysis for the behavioral sciences. second. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

