The role of local protein synthesis and degradation in axon regeneration
- PMID: 19520073
- PMCID: PMC2864402
- DOI: 10.1016/j.expneurol.2009.06.004
The role of local protein synthesis and degradation in axon regeneration
Abstract
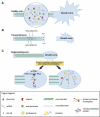
In axotomised regenerating axons, the first step toward successful regeneration is the formation of a growth cone. This requires a variety of dynamic morphological and biochemical changes in the axon, including the appearance of many new cytoskeletal, cell surface and signalling molecules. These changes suggest the activation of coordinated complex cellular processes. A recent development has been the demonstration that the regenerative ability of some axons depends on their capacity to locally synthesise new proteins and degrade others at the injury site autonomously from the cell body. There are also events involving the degradation of cytoskeletal and other molecules, and activation of signalling pathways, with axotomy-induced calcium changes probably being an initiating event. A future challenge will be to understand how this complex network of processes interacts in order to find therapeutic ways of promoting the regeneration of CNS axons.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
References
-
- Abrams T.W., Yovell Y., Onyike C.U., Cohen J.E., Jarrard H.E. Analysis of sequence-dependent interactions between transient calcium and transmitter stimuli in activating adenylyl cyclase in Aplysia: possible contribution to CS–US sequence requirement during conditioning. Learn Mem. 1998;4:496–509. - PubMed
-
- Alvarez J., Giuditta A., Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog. Neurobiol. 2000;62:1–62. - PubMed
-
- Aunis D., Bader M.F. The cytoskeleton as a barrier to exocytosis in secretory cells. J. Exp. Biol. 1988;139:253–266. - PubMed
-
- Bamburg J.R., McGough A., Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999;9:364–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

