Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones
- PMID: 19520167
- PMCID: PMC4603359
- DOI: 10.1016/j.mcn.2009.06.002
Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones
Abstract
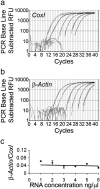
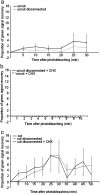
We have developed a compartmentalised culture model for the purification of axonal mRNA from embryonic, neonatal and adult rat dorsal root ganglia. This mRNA was used un-amplified for RT-qPCR. We assayed for the presence of axonal mRNAs encoding molecules known to be involved in axon growth and guidance. mRNAs for beta-actin, beta-tubulin, and several molecules involved in the control of actin dynamics and signalling during axon growth were found, but mRNAs for microtubule-associated proteins, integrins and cell surface adhesion molecules were absent. Quantification of beta-actin mRNA by means of qPCR showed that the transcript is present at the same level in embryonic, newborn and adult axons. Using the photoconvertible reporter Kaede we showed that there is local translation of beta-actin in axons, the rate being increased by axotomy. Knock down of beta-actin mRNA by RNAi inhibited the regeneration of new axon growth cones after in vitro axotomy, indicating that local translation of actin-related molecules is important for successful axon regeneration.
Figures







References
-
- Chierzi S, Ratto GM, Verma P, Fawcett JW. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur. J. Neurosci. 2005;21:2051–2062. - PubMed
-
- Chun JT, Gioio AE, Crispino M, Giuditta A, Kaplan BB. Differential compartmentalization of mRNAs in squid giant axon. J. Neurochem. 1996;67:1806–1812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
