Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition
- PMID: 19520830
- PMCID: PMC2695406
- DOI: 10.1073/pnas.0901462106
Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition
Abstract
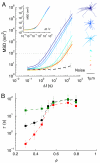
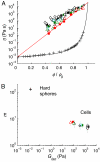
Mechanical robustness of the cell under different modes of stress and deformation is essential to its survival and function. Under tension, mechanical rigidity is provided by the cytoskeletal network; with increasing stress, this network stiffens, providing increased resistance to deformation. However, a cell must also resist compression, which will inevitably occur whenever cell volume is decreased during such biologically important processes as anhydrobiosis and apoptosis. Under compression, individual filaments can buckle, thereby reducing the stiffness and weakening the cytoskeletal network. However, the intracellular space is crowded with macromolecules and organelles that can resist compression. A simple picture describing their behavior is that of colloidal particles; colloids exhibit a sharp increase in viscosity with increasing volume fraction, ultimately undergoing a glass transition and becoming a solid. We investigate the consequences of these 2 competing effects and show that as a cell is compressed by hyperosmotic stress it becomes progressively more rigid. Although this stiffening behavior depends somewhat on cell type, starting conditions, molecular motors, and cytoskeletal contributions, its dependence on solid volume fraction is exponential in every instance. This universal behavior suggests that compression-induced weakening of the network is overwhelmed by crowding-induced stiffening of the cytoplasm. We also show that compression dramatically slows intracellular relaxation processes. The increase in stiffness, combined with the slowing of relaxation processes, is reminiscent of a glass transition of colloidal suspensions, but only when comprised of deformable particles. Our work provides a means to probe the physical nature of the cytoplasm under compression, and leads to results that are universal across cell type.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Elson EL. Cellular mechanics as an indicator of cytoskeletal structure and function. Ann Rev Biophys Chem. 1988;17:397–430. - PubMed
-
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. - PubMed
-
- Fabry B, et al. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87:148102. - PubMed
-
- Bursac P, et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4:557–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

