Hepatic CYP3A suppression by high concentrations of proteasomal inhibitors: a consequence of endoplasmic reticulum (ER) stress induction, activation of RNA-dependent protein kinase-like ER-bound eukaryotic initiation factor 2alpha (eIF2alpha)-kinase (PERK) and general control nonderepressible-2 eIF2alpha kinase (GCN2), and global translational shutoff
- PMID: 19520899
- PMCID: PMC2730383
- DOI: 10.1124/mol.109.056002
Hepatic CYP3A suppression by high concentrations of proteasomal inhibitors: a consequence of endoplasmic reticulum (ER) stress induction, activation of RNA-dependent protein kinase-like ER-bound eukaryotic initiation factor 2alpha (eIF2alpha)-kinase (PERK) and general control nonderepressible-2 eIF2alpha kinase (GCN2), and global translational shutoff
Retraction in
-
Notice of Retraction: Acharya P, Engel JC, Correia MA (2009) Hepatic CYP3A suppression by high concentrations of proteasomal inhibitors: A consequence of endoplasmic reticulum (ER) stress induction, activation of RNA-dependent protein kinase-like ER-bound eukaryotic initiation factor 2α (eIF2α)-kinase (PERK) and general control nonderepressible-2 eIF2α kinase (GCN2), and global translational shutoff. Mol Pharmacol 76(3):503-515; DOI: https://doi.org/10.1124/mol.109.056002.Mol Pharmacol. 2021 Aug;100(2):82. doi: 10.1124/mol.109.056002retraction. Mol Pharmacol. 2021. PMID: 34404737 Free PMC article. No abstract available.
Abstract
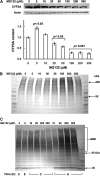
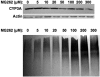
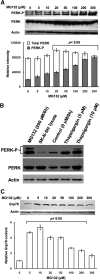
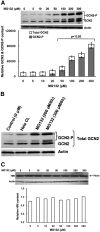
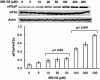
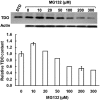
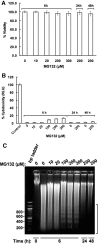
Hepatic cytochromes P450 3A (P450s 3A) are endoplasmic reticulum (ER)-proteins, responsible for xenobiotic metabolism. They are degraded by the ubiquitin-dependent 26S proteasome. Consistent with this, we have shown that proteasomal inhibitors N-benzoyloxycarbonyl (Z)-Leu-Leu-leucinal (MG132) and N-benzoyloxycarbonyl-Leu-Leu-Leu-B(OH)(2) (MG262) stabilize CYP3A proteins. However, MG132 has been reported to suppress P450s 3A as a result of impaired nuclear factor-kappaB activation and consequently reduced CYP3A protein stability. Because the MG132 concentration used in those studies was 10-fold higher than that required for CYP3A stabilization, we examined the effect of MG132 (0-300 microM) concentration-dependent proteasomal inhibition on CYP3A turnover in cultured primary rat hepatocytes. We found a biphasic MG132 concentration effect on CYP3A turnover: Stabilization at 5 to 10 muM with marked suppression at >100 microM. Proteasomal inhibitors reportedly induce ER stress, heat shock, and apoptotic response. At these high MG132 concentrations, such CYP3A suppression could be due to ER stress induction, so we monitored the activity of PERK [PKR (RNA-dependent protein kinase)-like ER kinase (EIF2AK3)], the ER stress-activated eukaryotic initiation factor 2alpha (eIF2alpha) kinase. Indeed, we found a marked (approximately 4-fold) MG132 concentration-dependent PERK autophosphorylation, along with an 8-fold increase in eIF2alpha-phosphorylation. In parallel, MG132 also activated GCN2 [general control nonderepressible-2 (EIF2AK4)] eIF2alpha kinase in a concentration-dependent manner, but not the heme-regulated inhibitor eIF2alpha kinase [(EIF2AK1)]. Pulse-chase, immunoprecipitation/immunoblotting analyses documented the consequently dramatic translational shutoff of total hepatic protein, including but not limited to CYP3A and tryptophan 2,3-dioxygenase protein syntheses. These findings reveal that at high concentrations, MG132 is indeed cytotoxic and can suppress CYP3A synthesis, a result confirmed by confocal immunofluorescence analyses of MG132-treated hepatocytes.
Figures



References
-
- Berlanga JJ, Santoyo J, and De Haro C (1999) Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem 265 754-762. - PubMed
-
- Bush KT, Goldberg AL, and Nigam SK (1997) Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem 272 9086-9092. - PubMed
-
- Correia MA, Sadeghi S, and Mundo-Paredes E (2005) Cytochrome P450 ubiquitination: branding for the proteolytic slaughter? Annu Rev Pharmacol Toxicol 45 439-464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

