Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae
- PMID: 19520922
- PMCID: PMC2848738
- DOI: 10.1165/rcmb.2007-0417OC
Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae
Abstract
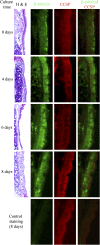
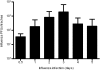
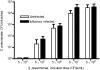
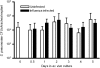
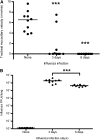
Influenza virus infections increase susceptibility to secondary bacterial infections, such as pneumococcal pneumonia, resulting in increased morbidity and mortality. Influenza-induced tissue damage is hypothesized to increase susceptibility to Streptococcus pneumoniae infection by increasing adherence to the respiratory epithelium. Using a mouse model of influenza infection followed by S. pneumoniae infection, we found that an influenza infection does not increase the number of pneumococci initially present within the trachea, but does inhibit pneumococcal clearance by 2 hours after infection. To determine whether influenza damage increases pneumococcal adherence, we developed a novel murine tracheal explant system to determine influenza-induced tissue damage and subsequent pneumococcal adherence. Murine tracheas were kept viable ex vivo as shown by microscopic examination of ciliary beating and cellular morphology using continuous media flow for up to 8 days. Tracheas were infected with influenza virus for 0.5-5 days ex vivo, and influenza-induced tissue damage and the early stages of repair to the epithelium were assessed histologically. A prior influenza infection did not increase pneumococcal adherence, even when the basement membrane was maximally denuded or during the repopulation of the basement membrane with undifferentiated epithelial cells. We measured mucociliary clearance in vivo and found it was decreased in influenza-infected mice. Together, our results indicate that exposure of the tracheal basement membrane contributes minimally to pneumococcal adherence. Instead, an influenza infection results in decreased tracheal mucociliary velocity and initial clearance of pneumococci, leading to an increased pneumococcal burden as early as 2 hours after pneumococcal infection.
Figures



Comment in
-
Inhibition of epithelial sodium channels and reduction of ciliary function in influenza.Am J Respir Cell Mol Biol. 2012 Mar;46(3):414. doi: 10.1165/ajrcmb.46.3.414. Am J Respir Cell Mol Biol. 2012. PMID: 22383654 No abstract available.
References
-
- Seki M, Yanagihara K, Higashiyama Y, Fukuda Y, Kaneko Y, Ohno H, Miyazaki Y, Hirakata Y, Tomono K, Kadota J, et al. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur Respir J 2004;24:143–149. - PubMed
-
- McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis 2004;190:519–526. - PubMed
-
- McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 2003;187:1000–1009. - PubMed
-
- McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 2002;186:341–350. - PubMed
-
- McCullers JA, Tuomanen EI. Molecular pathogenesis of pneumococcal pneumonia. Front Biosci 2001;6:D877–D889. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

