The role of the plasma membrane in the response of plant roots to aluminum toxicity
- PMID: 19521474
- PMCID: PMC2633877
- DOI: 10.4161/psb.1.2.2588
The role of the plasma membrane in the response of plant roots to aluminum toxicity
Abstract
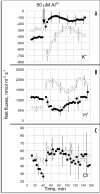
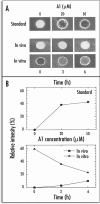
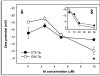
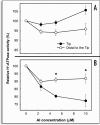
Al(3+), the predominant form of solubilized aluminum at pH values below 5.0, has been shown to exert a profound inhibitory effect on root elongation. Al is known to accumulate at the root apex. The plasma membrane represents the first potential target for Al toxicity, due to its pronounced binding to phospholipids. Al appears to alter both the structure and functions of the plasma membrane, and a great deal of research has been conducted concerning the interactions between Al and the plasma membrane. In this review, recent findings regarding the interactions between Al and the plasma membrane are described, specifically findings involving Al-induced alterations in the structure and function of the plasma membrane.
Keywords: acid soil; aluminum; plasma membrane; tolerance; toxicity.
Figures


References
-
- Baligar VC, Ahlrichs JL. Nature and distribution of acid soils in the world. In: Schaffert PE, editor. Proceeding of the Workshop to Develop a Strategy for Collaborative Research and Distribution of Technology in Sustainable Crop Production in Acid Savannas and other Problem Soils of the World. Purdue University; 1988. pp. 1–11.
-
- Hertwell B, Pember FR. The presence of aluminum as a reason for the difference in the effect of so-called acid soil on barley and rye. Soil Sci. 1918;6:259–279.
-
- Matsumoto H. Cell biology of Al tolerance and toxicity in higher plants. Int Rev Cytol. 2000;200:1–40. - PubMed
-
- Kochia LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260.
-
- Haug A, Caldwell CR. Aluminum toxicity in plants: The role of root plasma membrane and calmodulin. In: St John JB, Elliot B, Jacson PC, editors. Frontiers of Membrane Research in Agriculture (Beltsville Symposium 9) Totowa: Rowman and Allanheld; 1985. pp. 359–381.
LinkOut - more resources
Full Text Sources
Miscellaneous
