Directed mammalian gene regulatory networks using expression and comparative genomic hybridization microarray data from radiation hybrids
- PMID: 19521529
- PMCID: PMC2690838
- DOI: 10.1371/journal.pcbi.1000407
Directed mammalian gene regulatory networks using expression and comparative genomic hybridization microarray data from radiation hybrids
Abstract
Meiotic mapping of quantitative trait loci regulating expression (eQTLs) has allowed the construction of gene networks. However, the limited mapping resolution of these studies has meant that genotype data are largely ignored, leading to undirected networks that fail to capture regulatory hierarchies. Here we use high resolution mapping of copy number eQTLs (ceQTLs) in a mouse-hamster radiation hybrid (RH) panel to construct directed genetic networks in the mammalian cell. The RH network covering 20,145 mouse genes had significant overlap with, and similar topological structures to, existing biological networks. Upregulated edges in the RH network had significantly more overlap than downregulated. This suggests repressive relationships between genes are missed by existing approaches, perhaps because the corresponding proteins are not present in the cell at the same time and therefore unlikely to interact. Gene essentiality was positively correlated with connectivity and betweenness centrality in the RH network, strengthening the centrality-lethality principle in mammals. Consistent with their regulatory role, transcription factors had significantly more outgoing edges (regulating) than incoming (regulated) in the RH network, a feature hidden by conventional undirected networks. Directed RH genetic networks thus showed concordance with pre-existing networks while also yielding information inaccessible to current undirected approaches.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
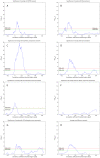
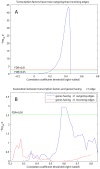
 values over results from A to G. One-sided Fisher's exact and chi-square tests used to assess overlap significance.
values over results from A to G. One-sided Fisher's exact and chi-square tests used to assess overlap significance.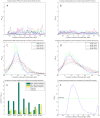
 values for overlap between randomly permuted RH networks and different existing datasets (HPRD, KEGG, SymAtlas coexpression, GO, GO-molecular function, GO-cellular component and GO-biological process annotation networks). (C) Overlap between RH networks constructed from a subset of randomly selected RH clones and HPRD network. Mean of overlap significance (solid line) over 50 random subsets shown with standard errors calculated by bootstrapping (dash-dot line). (D) Same as (C) except averaged
values for overlap between randomly permuted RH networks and different existing datasets (HPRD, KEGG, SymAtlas coexpression, GO, GO-molecular function, GO-cellular component and GO-biological process annotation networks). (C) Overlap between RH networks constructed from a subset of randomly selected RH clones and HPRD network. Mean of overlap significance (solid line) over 50 random subsets shown with standard errors calculated by bootstrapping (dash-dot line). (D) Same as (C) except averaged  values over different existing datasets. (E) Comparing different thresholding approaches. Maximum
values over different existing datasets. (E) Comparing different thresholding approaches. Maximum  over varying correlation coefficient thresholds shown. (F) Comparing betweenness centralities of RH and HPRD networks. P-values of Spearman correlation coefficients (one-sided, positive direction) between the betweenness centralities of RH and HPRD networks shown.
over varying correlation coefficient thresholds shown. (F) Comparing betweenness centralities of RH and HPRD networks. P-values of Spearman correlation coefficients (one-sided, positive direction) between the betweenness centralities of RH and HPRD networks shown.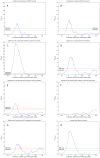
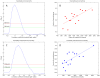
References
-
- Vidal M. A biological atlas of functional maps. Cell. 2001;104:333–339. - PubMed
-
- Ge H, Walhout AJ, Vidal M. Integrating ‘omic’ information: a bridge between genomics and systems biology. Trends Genet. 2003;10:551–560. - PubMed
-
- Stuart JM, Segal E, Koller D, Kim SK. A gene-coexpression network for global discovery of conserved genetic modules. Science. 2003;302:249–255. - PubMed
-
- Cusick ME, Klitgord N, Vidal M, Hill DE. Interactome: gateway into systems biology. Human Molecular Genetics. 2005;14:R171–R181. - PubMed
-
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. - PubMed

