Behavioral modulation of stimulus-evoked oscillations in barrel cortex of alert rats
- PMID: 19521539
- PMCID: PMC2694660
- DOI: 10.3389/neuro.07.010.2009
Behavioral modulation of stimulus-evoked oscillations in barrel cortex of alert rats
Abstract
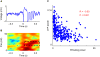
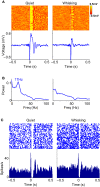
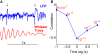
Stimulus-evoked oscillations have been observed in the visual, auditory, olfactory and somatosensory systems. To further our understanding of these oscillations, it is essential to study their occurrence and behavioral modulation in alert, awake animals. Here we show that microstimulation in barrel cortex of alert rats evokes 15-18 Hz oscillations that are strongly modulated by motor behavior. In freely whisking rats, we found that the power of the microstimulation-evoked oscillation in the local field potential was inversely correlated to the strength of whisking. This relationship was also present in rats performing a stimulus detection task suggesting that the effect was not due to sleep or drowsiness. Further, we present a computational model of the thalamocortical loop which recreates the observed phenomenon and predicts some of its underlying causes. These findings demonstrate that stimulus-evoked oscillations are strongly influenced by motor modulation of afferent somatosensory circuits.
Keywords: behavioral modulation; cortical microstimulation; evoked oscillations; thalamocortical.
Figures




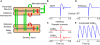
References
-
- Bartley H. S., Bishop G. H. (1932). The cortical response to stimulation of the optic nerve in the rabbit. Am. J. Physiol. 103, 159–172
LinkOut - more resources
Full Text Sources

