Circulating osteogenic precursor cells in heterotopic bone formation
- PMID: 19522009
- PMCID: PMC3496263
- DOI: 10.1002/stem.150
Circulating osteogenic precursor cells in heterotopic bone formation
Abstract
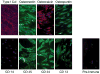
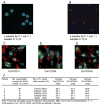
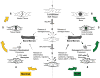
Cells with osteogenic potential can be found in a variety of tissues. Here we show that circulating osteogenic precursor (COP) cells, a bone marrow-derived type I collagen+/CD45+ subpopulation of mononuclear adherent cells, are present in early preosseous fibroproliferative lesions in patients with fibrodysplasia ossificans progressiva (FOP) and nucleate heterotopic ossification (HO) in a murine in vivo implantation assay. Blood samples from patients with FOP with active episodes of HO contain significantly higher numbers of clonally derived COP cell colonies than patients with stable disease or unaffected individuals. The highest level of COP cells was found in a patient just before the clinical onset of an HO exacerbation. Our studies show that even COP cells derived from an unaffected individual can contribute to HO in genetically susceptible host tissue. The possibility that circulating, hematopoietic-derived cells with osteogenic potential can seed inflammatory sites has tremendous implications and, to our knowledge, represents the first example of their involvement in clinical HO. Thus, bone formation is not limited to cells of the mesenchymal lineage, and circulating cells of hematopoietic origin can also serve as osteogenic precursors at remote sites of tissue inflammation.
Conflict of interest statement
Figures



References
-
- Huss R, Lange C, Weissinger EM, Kolb HJTK. Evidence of peripheral blood-derived, plastic-adherent CD34(−/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells. 2000;18:252–260. - PubMed
-
- Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. The International Journal of Biochemistry & Cell Biology. 2004;36:585–597. - PubMed
-
- Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–45. - PubMed
-
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

