Generation of dopamine neurons with improved cell survival and phenotype maintenance using a degradation-resistant nurr1 mutant
- PMID: 19522012
- PMCID: PMC2816355
- DOI: 10.1002/stem.146
Generation of dopamine neurons with improved cell survival and phenotype maintenance using a degradation-resistant nurr1 mutant
Abstract
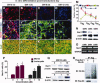
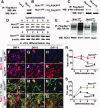
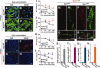
Nurr1 is a transcription factor specific for the development and maintenance of the midbrain dopamine (DA) neurons. Exogenous Nurr1 in neural precursor (NP) cells induces the differentiation of DA neurons in vitro that are capable of reversing motor dysfunctions in a rodent model for Parkinson disease. The promise of this therapeutic approach, however, is unclear due to poor cell survival and phenotype loss of DA cells after transplantation. We herein demonstrate that Nurr1 proteins undergo ubiquitin-proteasome-system-mediated degradation in differentiating NP cells. The degradation process is activated by a direct Akt-mediated phosphorylation of Nurr1 proteins and can be prevented by abolishing the Akt-target sequence in Nurr1 (Nurr1(Akt)). Overexpression of Nurr1(Akt) in NP cells yielded DA neurons in which Nurr1 protein levels were maintained for prolonged periods. The sustained Nurr1 expression endowed the Nurr1(Akt)-induced DA neurons with resistance to toxic stimuli, enhanced survival, and sustained DA phenotypes in vitro and in vivo after transplantation.
Figures


Similar articles
-
NURR1 in Parkinson disease--from pathogenesis to therapeutic potential.Nat Rev Neurol. 2013 Nov;9(11):629-36. doi: 10.1038/nrneurol.2013.209. Epub 2013 Oct 15. Nat Rev Neurol. 2013. PMID: 24126627 Review.
-
Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival.Stem Cells. 2010 Mar 31;28(3):501-12. doi: 10.1002/stem.294. Stem Cells. 2010. PMID: 20049900
-
Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression.Stem Cells. 2007 May;25(5):1252-62. doi: 10.1634/stemcells.2006-0274. Epub 2007 Jan 18. Stem Cells. 2007. PMID: 17234994
-
Differential actions of the proneural genes encoding Mash1 and neurogenins in Nurr1-induced dopamine neuron differentiation.J Cell Sci. 2006 Jun 1;119(Pt 11):2310-20. doi: 10.1242/jcs.02955. J Cell Sci. 2006. PMID: 16723737
-
Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells.Cell Tissue Res. 2004 Oct;318(1):45-52. doi: 10.1007/s00441-004-0974-7. Epub 2004 Sep 1. Cell Tissue Res. 2004. PMID: 15340833 Review.
Cited by
-
The Critical Role of Nurr1 as a Mediator and Therapeutic Target in Alzheimer's Disease-related Pathogenesis.Aging Dis. 2020 May 9;11(3):705-724. doi: 10.14336/AD.2019.0718. eCollection 2020 May. Aging Dis. 2020. PMID: 32489714 Free PMC article. Review.
-
Telencephalin protects PAJU cells from amyloid beta protein-induced apoptosis by activating the ezrin/radixin/moesin protein family/phosphatidylinositol-3-kinase/protein kinase B pathway.Neural Regen Res. 2012 Oct 5;7(28):2189-98. doi: 10.3969/j.issn.1673-5374.2012.028.004. Neural Regen Res. 2012. PMID: 25538739 Free PMC article.
-
α-Synuclein Induces the GSK-3-Mediated Phosphorylation and Degradation of NURR1 and Loss of Dopaminergic Hallmarks.Mol Neurobiol. 2021 Dec;58(12):6697-6711. doi: 10.1007/s12035-021-02558-9. Epub 2021 Oct 5. Mol Neurobiol. 2021. PMID: 34609698 Free PMC article.
-
NURR1 in Parkinson disease--from pathogenesis to therapeutic potential.Nat Rev Neurol. 2013 Nov;9(11):629-36. doi: 10.1038/nrneurol.2013.209. Epub 2013 Oct 15. Nat Rev Neurol. 2013. PMID: 24126627 Review.
-
PIASγ enhanced SUMO-2 modification of Nurr1 activation-function-1 domain limits Nurr1 transcriptional synergy.PLoS One. 2013;8(1):e55035. doi: 10.1371/journal.pone.0055035. Epub 2013 Jan 24. PLoS One. 2013. PMID: 23358114 Free PMC article.
References
-
- Zetterstrom RH, Solomin L, Jansson L, et al. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. - PubMed
-
- Eells JB, Lipska BK, Yeung SK, et al. Nurr1-null heterozygous mice have reduced mesolimbic and mesocortical dopamine levels and increased stress-induced locomotor activity. Behav Brain Res. 2002;136:267–275. - PubMed
-
- Wallen A, Zetterstrom RH, Solomin L, et al. Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp Cell Res. 1999;253:737–746. - PubMed
-
- Sousa KM, Mira H, Hall AC, et al. Microarray analyses support a role for Nurr1 in resistance to oxidative stress and neuronal differentiation in neural stem cells. Stem Cells. 2007;25:511–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

