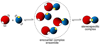
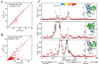
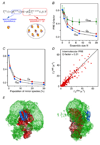
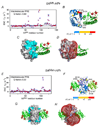
Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes
- PMID: 19522502
- PMCID: PMC2825090
- DOI: 10.1021/cr900033p
Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes
Figures





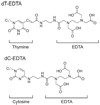
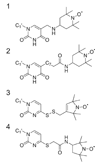
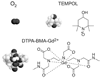

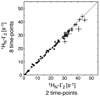


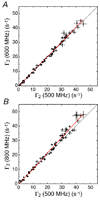



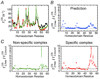

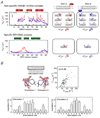



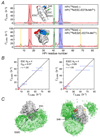
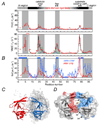
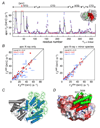
References
-
- Branden C, Tooze J. Introduction to Protein Structure. 2nd ed. New York: Garland Publishing Inc; 1998.
-
- Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Proteins. 1995;21:167. - PubMed
-
- Onuchic JN, Luthey-Schulten Z, Wolynes PG. Annu. Rev. Phys. Chem. 1997;48:545. - PubMed
-
- Miyashita O, Wolynes PG, Onuchic JN. J. Phys. Chem. B. 2005;109:1959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

