BAFF is increased in renal transplant patients following treatment with alemtuzumab
- PMID: 19522878
- PMCID: PMC4876605
- DOI: 10.1111/j.1600-6143.2009.02710.x
BAFF is increased in renal transplant patients following treatment with alemtuzumab
Abstract
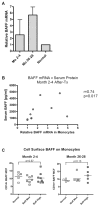
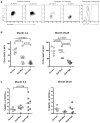
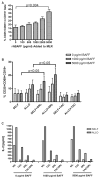
Alemtuzumab is a monoclonal antibody that depletes T and B cells and is used as induction therapy for renal transplant recipients. Without long-term calcineurin inhibitor (CNI) therapy, alemtuzumab-treated patients have a propensity to develop alloantibody and may undergo antibody-mediated rejection (AMR). In pursuit of a mechanistic explanation, we analyzed peripheral B cells and serum of these patients for BAFF (Blys) and BAFF-R, factors known to be integral for B-cell activation, survival, and homeostasis. Serum BAFF levels of 22/24 alemtuzumab-treated patients were above normal range, with average levels of 1967 pg/mL compared to 775 pg/mL in healthy controls (p = 0.006). BAFF remained elevated 2 years posttransplant in 78% of these patients. BAFF-R on CD19(+) B cells was significantly downregulated, suggesting ligand/receptor engagement. BAFF mRNA expression was increased 2-7-fold in CD14(+) cells of depleted patients, possibly linking monocytes to the BAFF dysregulation. Addition of recombinant BAFF to mixed lymphocyte cultures increased B-cell activation to alloantigen, as measured by CD25 and CD69 coexpression on CD19(+) cells. Of note, addition of sirolimus (SRL) augmented BAFF-enhanced B-cell activation whereas CNIs blocked it. These data suggest associations between BAFF/BAFF-R and AMR in alemtuzumab-treated patients.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
-
- Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81:81–87. - PubMed
-
- Noris M, Casiraghi F, Todeschini M, et al. Regulatory T cells and T cell depletion: Role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–1018. - PubMed
-
- Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. - PubMed
-
- Cai J, Terasaki PI, Bloom DD, et al. Correlation between human leukocyte antigen antibody production and serum creatinine in patients receiving sirolimus monotherapy after Campath-1H induction. Transplantation. 2004;78:919–924. - PubMed
-
- Knechtle SJ, Pirsch JD, Fechner H, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant. 2003;3:722–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

