Lysosomal localization of GLUT8 in the testis--the EXXXLL motif of GLUT8 is sufficient for its intracellular sorting via AP1- and AP2-mediated interaction
- PMID: 19523115
- PMCID: PMC2730553
- DOI: 10.1111/j.1742-4658.2009.07089.x
Lysosomal localization of GLUT8 in the testis--the EXXXLL motif of GLUT8 is sufficient for its intracellular sorting via AP1- and AP2-mediated interaction
Abstract
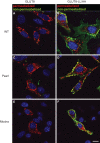
The class III sugar transport facilitator GLUT8 co-localizes with the lysosomal protein LAMP1 in heterologous expression systems. GLUT8 carries a [D/E]XXXL[L/I]-type dileucine sorting signal that has been postulated to retain the protein in an endosomal/lysosomal compartment via interactions with clathrin adaptor protein (AP) complexes. However, contradictory findings have been described regarding the subcellular localization of the endogenous GLUT8 and the adaptor proteins that interact with its dileucine motif. Here we demonstrate that endogenous GLUT8 is localized in a late endosomal/lysosomal compartment of spermatocytes and spermatids, and that the adaptor complexes AP1 and AP2, but not AP3 or AP4, interact with its N-terminal intracellular domain (NICD). In addition, fusion of the GLUT8 NICD to the tailless lumenal domain of the IL-2 receptor alpha chain (TAC) protein (interleukin-2 receptor a chain) targeted the protein to intracellular membranes, indicating that its N-terminal dileucine signal is sufficient for endosomal/lysosomal targeting of the transporter. The localization and targeting of GLUT8 show striking similarities to sorting mechanisms reported for lysosomal proteins. Therefore, we suggest a potential role for GLUT8 in the so far unexplored substrate transport across intracellular membranes.
Figures





References
-
- Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004;28:364–371. - PubMed
-
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol Membr Biol. 2001;18:247–256. - PubMed
-
- Augustin R, Riley J, Moley KH. GLUT8 contains a [DE]XXXL[LI] sorting motif and localizes to a late endosomal/lysosomal compartment. Traffic. 2005;6:1196–1212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

