Alternative infectious entry pathways for dengue virus serotypes into mammalian cells
- PMID: 19523154
- PMCID: PMC7162254
- DOI: 10.1111/j.1462-5822.2009.01345.x
Alternative infectious entry pathways for dengue virus serotypes into mammalian cells
Abstract
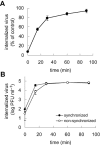
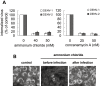
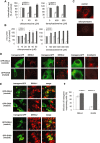
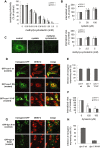
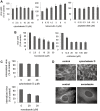
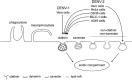
The entry of two dengue virus (DENV) serotypes into Vero cells was analysed using biochemical inhibitors, dominant negative mutants of cellular proteins involved in endocytic pathways, fluorescence microscopy and infectivity determinations. By treatment with dansylcadaverine and chlorpromazine and overexpression of a dominant negative form of the Eps15 protein, a clathrin-mediated endocytosis for productive DENV-1 internalization into Vero cells was demonstrated whereas the infectious entry of DENV-2 in the same cell system was independent of clathrin. Treatment with the inhibitors nystatin and methyl-beta-cyclodextrin, as well as transfection of Vero cells with dominant negative caveolin-1, had no effect on DENV-2 virus infection. It was also shown, by using the K44A mutant and the inhibitor dynasore, that dynamin was required for DENV-2 entry. Consequently, the infectious entry of DENV-2 into Vero cells occurs by a non-classical endocytic pathway independent of clathrin, caveolae and lipid rafts, but dependent on dynamin. By contrast, DENV-2 entry into A549 cells was clathrin-dependent, as previously reported in HeLa, C6/36 and BS-C-1 cells. Our results conclusively show, for the first time, a differential mode of infective entry for DENV-1 and DENV-2 into a common host cell, Vero cells, as well as alternative entry pathways for a given serotype, DENV-2, into different types of cells.
Figures

References
-
- Acosta, E.G. , Talarico, L.B. , and Damonte, E.B. (2008a) Cell entry of dengue virus. Future Virol 3: 471–479.
-
- Acosta, E.G. , Castilla, V. , and Damonte, E.B. (2008b) Functional entry of dengue virus into Aedes albopictus mosquito cells is dependent on clathrin‐mediated endocytosis. J Gen Virol 89: 474–484. - PubMed
-
- Altmeyer, R. (2004) Virus attachment and entry offer numerous targets for antiviral therapy. Curr Pharm Des 10: 3701–3712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

