The effect of mimicking febrile temperature and drug stress on malarial development
- PMID: 19523215
- PMCID: PMC2707362
- DOI: 10.1186/1476-0711-8-19
The effect of mimicking febrile temperature and drug stress on malarial development
Abstract
Background: Malaria remains one of the most important tropical diseases of human with 1-2 million deaths annually especially caused by P. falciparum. During malarial life cycle, they exposed to many environmentally stresses including wide temperature fluctuation and pharmacological active molecules. These trigger malarial evolutionarily adaptive responses. The effect of febrile temperature on malarial growth, development and drug susceptibility by mimicking patient in treatment failure before and after drug uptake was examined.
Methods: Sensitivities of P. falciparum to antimalarial drug (chloroquine, mefloquine, quinine and artesunate) were investigated based on the incorporation of [3H] hypoxanthine into parasite nucleic acids or radioisotopic technique. The number of parasites was examined under microscope following Giemsa staining and the parasite development at the end of each phase was counted and comparison of parasite number was made. The proteome was separated, blotted and hybridized with anti-Hsp70s primary antibody. The hybridized proteins were separately digested with trypsin and identified by MALDI-TOF peptide mass fingerprint.
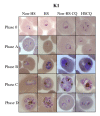
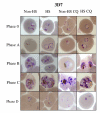
Results: The results show that febrile temperature is capable of markedly inhibiting the growth of field isolate P. falciparum but not to K1 and 3D7 standard strains. K1 and 3D7 grown under heat shock developed greater and the reinfection rate was increased up to 2-folds when compared to that of non-heat shock group. The IC50 value of K1 toward chloroquine, mefloquine and quinine under heat shock was higher than that of K1 under non-heat shock which is opposite to that of 3D7. Heat shock caused death in field isolated parasite. It was also found that the febrile temperature coped with chloroquine uptake had no effect to the development, drug sensitivity and the parasite number of K1 strain. In the opposite way, heat shock and chloroquine shows extremely effect toward 3D7 and field isolate PF91 as shown by higher number of dead parasites compared to that of control group. After culture under high temperature with artesunate, the total parasite number of all strains including K1, 3D7 and PF91 was extremely decreased and the parasite was not found at the end. Additionally, the expression of pfHsp70s was found in all strains and conditions as shown in 120 kDa hybridized band. However, the proteome extracted from K1 grown under heat shock with chloroquine, anti-pfHsp70 interacted with additional three bands identified by MALDI-TOF as elongation factor-1alpha (83 kDa), pfHsp86 (60 kDa) and phosphoethanolamine N-methyltransferase (43 kDa).
Conclusion: In conclusion, febrile temperature was capable of markedly inhibiting the growth of field isolate P. falciparum while the development, reinfection rate and drug (chloroquine, mefloquine and quinine) resistant level of standard strain K1 was enhanced. However, the febrile temperature coped with chloroquine had no effect to the development, drug sensitivity and the parasite number of K1 strain. In the opposite way, heat shock and chloroquine showed extremely effect toward 3D7 and field isolate PF91 as shown by some died parasites. Heat shock protein 70 (pfHSP70) of strain K1 under heat shock with chloroquine might involved in many pathways in order to sustain the parasite.
Figures







Similar articles
-
[In vitro susceptibility of P. falciparum isolates from Abidjan (Côte d'Ivoire) to quinine, artesunate and chloroquine].Sante. 2008 Jan-Mar;18(1):43-7. doi: 10.1684/san.2008.0103. Sante. 2008. PMID: 18684691 French.
-
In vitro susceptibility of Indian Plasmodium falciparum isolates to different antimalarial drugs & antibiotics.Indian J Med Res. 2017 Nov;146(5):622-628. doi: 10.4103/ijmr.IJMR_1688_15. Indian J Med Res. 2017. PMID: 29512604 Free PMC article.
-
Ex vivo susceptibility of Plasmodium falciparum isolates from Dakar, Senegal, to seven standard anti-malarial drugs.Malar J. 2011 Oct 20;10:310. doi: 10.1186/1475-2875-10-310. Malar J. 2011. PMID: 22014157 Free PMC article.
-
The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum.Fundam Clin Pharmacol. 2003 Apr;17(2):147-53. doi: 10.1046/j.1472-8206.2003.00164.x. Fundam Clin Pharmacol. 2003. PMID: 12667224 Review.
-
Plasmodium falciparum heat shock proteins as antimalarial drug targets: An update.Cell Stress Chaperones. 2024 Apr;29(2):326-337. doi: 10.1016/j.cstres.2024.03.007. Epub 2024 Mar 20. Cell Stress Chaperones. 2024. PMID: 38518861 Free PMC article. Review.
Cited by
-
Single-Cell RNA Sequencing Reveals Cellular Heterogeneity and Stage Transition under Temperature Stress in Synchronized Plasmodium falciparum Cells.Microbiol Spectr. 2021 Sep 3;9(1):e0000821. doi: 10.1128/Spectrum.00008-21. Epub 2021 Jul 7. Microbiol Spectr. 2021. PMID: 34232098 Free PMC article.
-
A novel flow cytometric hemozoin detection assay for real-time sensitivity testing of Plasmodium falciparum.PLoS One. 2013 Apr 24;8(4):e61606. doi: 10.1371/journal.pone.0061606. Print 2013. PLoS One. 2013. PMID: 23637865 Free PMC article.
-
The Role of Hsp70s in the Development and Pathogenicity of Plasmodium falciparum.Adv Exp Med Biol. 2021;1340:75-95. doi: 10.1007/978-3-030-78397-6_3. Adv Exp Med Biol. 2021. PMID: 34569021
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

