Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones
- PMID: 19524440
- PMCID: PMC2844727
- DOI: 10.1016/j.tcb.2009.03.009
Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones
Abstract
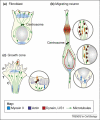
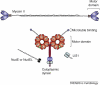
Motor proteins are involved in a wide range of cellular and subcellular movements. Recent studies have implicated two motor proteins in particular, myosin II and cytoplasmic dynein, in diverse aspects of cell migration. This review focuses on emerging roles for these proteins in the nervous system, with particular emphasis on migrating neurons and neuronal growth cones. The former cells exhibit unusual features of centrosome and nuclear movement, whereas growth cones offer an opportunity to evaluate motor protein function in a region of cytoplasm free of these organelles.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

