Autophagy suppresses tumorigenesis through elimination of p62
- PMID: 19524509
- PMCID: PMC2802318
- DOI: 10.1016/j.cell.2009.03.048
Autophagy suppresses tumorigenesis through elimination of p62
Erratum in
- Cell. 2011 Apr 15;145(2):322
Abstract
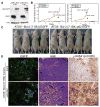
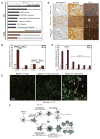
Allelic loss of the essential autophagy gene beclin1 occurs in human cancers and renders mice tumor-prone suggesting that autophagy is a tumor-suppression mechanism. While tumor cells utilize autophagy to survive metabolic stress, autophagy also mitigates the resulting cellular damage that may limit tumorigenesis. In response to stress, autophagy-defective tumor cells preferentially accumulated p62/SQSTM1 (p62), endoplasmic reticulum (ER) chaperones, damaged mitochondria, reactive oxygen species (ROS), and genome damage. Moreover, suppressing ROS or p62 accumulation prevented damage resulting from autophagy defects indicating that failure to regulate p62 caused oxidative stress. Importantly, sustained p62 expression resulting from autophagy defects was sufficient to alter NF-kappaB regulation and gene expression and to promote tumorigenesis. Thus, defective autophagy is a mechanism for p62 upregulation commonly observed in human tumors that contributes directly to tumorigenesis likely by perturbing the signal transduction adaptor function of p62-controlling pathways critical for oncogenesis.
Figures
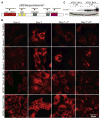
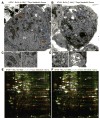

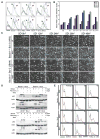
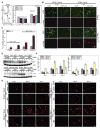
References
-
- Balajee AS, Geard CR. Replication protein A and gamma-H2AX foci assembly is triggered by cellular response to DNA double-strand breaks. Exp Cell Res. 2004;300:320–334. - PubMed
-
- Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. J Biol Chem. 2002;277:14127–14134. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

