Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation
- PMID: 19524513
- PMCID: PMC2727676
- DOI: 10.1016/j.cell.2009.05.037
Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation
Abstract
Programmed necrosis is a form of caspase-independent cell death whose molecular regulation is poorly understood. The kinase RIP1 is crucial for programmed necrosis, but also mediates activation of the prosurvival transcription factor NF-kappaB. We postulated that additional molecules are required to specifically activate programmed necrosis. Using a RNA interference screen, we identified the kinase RIP3 as a crucial activator for programmed necrosis induced by TNF and during virus infection. RIP3 regulates necrosis-specific RIP1 phosphorylation. The phosphorylation of RIP1 and RIP3 stabilizes their association within the pronecrotic complex, activates the pronecrotic kinase activity, and triggers downstream reactive oxygen species production. The pronecrotic RIP1-RIP3 complex is induced during vaccinia virus infection. Consequently, RIP3(-/-) mice exhibited severely impaired virus-induced tissue necrosis, inflammation, and control of viral replication. Our findings suggest that RIP3 controls programmed necrosis by initiating the pronecrotic kinase cascade, and that this is necessary for the inflammatory response against virus infections.
Figures

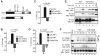
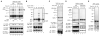
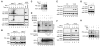



Comment in
-
RIP kinases at the crossroads of cell death and survival.Cell. 2009 Jul 23;138(2):229-32. doi: 10.1016/j.cell.2009.07.006. Cell. 2009. PMID: 19632174 Review.
References
-
- Adams S, Pankow S, Werner S, Munz B. Regulation of NF-κB activity and keratinocyte differentiation by the RIP4 protein: implications for cutaneous wound repair. J Invest Dermatol. 2007;127:538–544. - PubMed
-
- Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol. 2002;3:1013–1018. - PubMed
-
- Cauwels A, Janssen B, Waeytens A, Cuvelier C, Brouckaert P. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat Immunol. 2003;4:387–393. - PubMed
-
- Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

