Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy?
- PMID: 19524522
- PMCID: PMC2748990
- DOI: 10.1016/j.neuron.2009.05.015
Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy?
Abstract
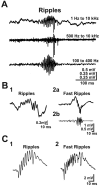
Brain circuits oscillate during sleep. The same circuits appear to generate pathological oscillations. In this review, we discuss recent advances in our understanding of how epilepsy co-opts normal, sleep-related circuits to generate seizures.
Figures

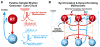
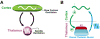




References
-
- Alzheimer C, Sutor B, ten Bruggencate G. Transient and selective blockade of adenosine A1-receptors by 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) causes sustained epileptiform activity in hippocampal CA3 neurons of guinea pigs. Neuroscience Letters. 1989;99:107–112. - PubMed
-
- Alzheimer C, Sutor B, Ten Bruggencate G. Disinhibition of hippocampal CA3 neurons induced by suppression of an adenosine A1 receptor-mediated inhibitory tonus: Pre- and postsynaptic components. Neuroscience. 1993;57:565–575. - PubMed
-
- Andersen P, Andersson S, Junge K, Sveen O. Xth Scandinavian EEG meeting: Voksenåsen, Oslo, Norway March 10-11, 1967 Secretary: C. W. Sem-Jacobsen The EEG Research Institute, Gaustad Sykehus, Oslo 3 (Norway) Electroencephalography and Clinical Neurophysiology. 1968;24:87–92.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

