Retinoid signaling and neurogenin2 function are coupled for the specification of spinal motor neurons through a chromatin modifier CBP
- PMID: 19524524
- PMCID: PMC2705669
- DOI: 10.1016/j.neuron.2009.04.025
Retinoid signaling and neurogenin2 function are coupled for the specification of spinal motor neurons through a chromatin modifier CBP
Abstract
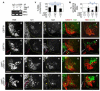
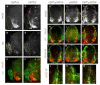
Extracellular signals and cell-intrinsic transcription factors cooperatively instruct generation of diverse neurons. However, little is known about how neural progenitors integrate both cues and orchestrate chromatin changes for neuronal specification. Here, we report that extrinsic signal retinoic acid (RA) and intrinsic transcription factor Neurogenin2 (Ngn2) collaboratively trigger transcriptionally active chromatin in spinal motor neuron genes during development. Retinoic acid receptor (RAR) binds Ngn2 and is thereby recruited to motor neuron genes targeted by Ngn2. RA then facilitates the recruitment of a histone acetyltransferase CBP to the Ngn2/RAR-complex, markedly inducing histone H3/H4-acetylation. Correspondingly, timely inactivation of CBP and its paralog p300 results in profound defects in motor neuron specification and motor axonal projection, accompanied by significantly reduced histone H3-acetylation of the motor neuron enhancer. Our study uncovers the mechanism by which extrinsic RA-signal and intrinsic transcription factor Ngn2 cooperate for cell fate specification through their synergistic activity to trigger transcriptionally active chromatin.
Figures


References
-
- Appel B, Eisen JS. Retinoids run rampant: multiple roles during spinal cord and motor neuron development. Neuron. 2003;40:461–464. - PubMed
-
- Arany Z, Newsome D, Oldread E, Livingston DM, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. - PubMed
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. - PubMed
-
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. - PubMed
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

