Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice
- PMID: 19524633
- PMCID: PMC2949440
- DOI: 10.1016/j.humimm.2009.06.005
Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice
Abstract
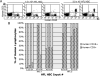
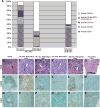
Immunodeficient mice bearing components of a human immune system present a novel approach for studying human immune responses. We investigated the number, phenotype, developmental kinetics, and function of developing human immune cells following transfer of CD34(+) hematopoietic stem cell (HSC) preparations originating from second trimester human fetal liver (HFL), umbilical cord blood (UCB), or granulocyte colony-stimulating factor-mobilized adult blood (G-CSF-AB) delivered via intrahepatic injection into sublethally irradiated neonatal NOD-scid/gammac(-/-), Balb/c-Rag1(-/-)gammac(-/-), and C.B-17-scid/bg mice. HFL and UCB HSC provided the greatest number and breadth of developing cells. NOD-scid/gammac(-/-) and Balb/c-Rag1(-/-)gammac(-/-) harbored human B and dendritic cells as well as human platelets in peripheral blood, whereas NOD-scid/gammac(-/-) mice harbored higher levels of human T cells. NOD-scid/gammac(-/-) mice engrafted with HFL CD34(+) HSC demonstrated human immunological competence evidenced by white pulp expansion and increases in total human immunoglobulin following immunization with T-dependent antigens and delayed-type hypersensitivity-infiltrating leukocytes in response to antigenic challenge. In conclusion, we describe an encouraging base system for studying human hematopoietic lineage development and function utilizing human HFL or UCB HSC-engrafted NOD-scid/gammac(-/-) mice that is well suited for future studies toward the development of a fully competent humanized mouse model.
Figures





References
-
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006 Nov;12(11):1316–1322. - PubMed
-
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007 Feb;7(2):118–130. - PubMed
-
- Pober JS, Bothwell AL, Lorber MI, McNiff JM, Schechner JS, Tellides G. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003 Sep;25(2):167–80. - PubMed
-
- Banuelos SJ, Shultz LD, Greiner DL, Burzenski LM, Gott B, Lyons BL, et al. Rejection of human islets and human HLA-A2.1 transgenic mouse islets by alloreactive human lymphocytes in immunodeficient NOD-scid and NOD-Rag1(null)Prf1(null) mice. Clin Immunol. 2004 Sep;112(3):273–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

