Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces
- PMID: 19524784
- PMCID: PMC2697127
- DOI: 10.1016/j.imlet.2009.03.013
Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces
Abstract
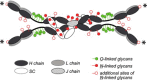
An explanation of the principles and mechanisms involved in peaceful co-existence between animals and the huge, diverse, and ever-changing microbiota that resides on their mucosal surfaces represents a challenging puzzle that is fundamental in everyday survival. In addition to mechanical barriers and a variety of innate defense factors, mucosal immunoglobulins (Igs) provide protection by two complementary mechanisms: specific antibody activity and innate, Ig glycan-mediated binding, both of which serve to contain the mucosal microbiota in its physiological niche. Thus, the interaction of bacterial ligands with IgA glycans constitutes a discrete mechanism that is independent of antibody specificity and operates primarily in the intestinal tract. This mucosal site is by far the most heavily colonized with an enormously diverse bacterial population, as well as the most abundant production site for antibodies, predominantly of the IgA isotype, in the entire immune system. In embodying both adaptive and innate immune mechanisms within a single molecule, S-IgA maintains comprehensive protection of mucosal surfaces with economy of structure and function.
Figures
References
-
- Abraham S.N., Bishop B.L., Sharon N., Ofek I. Adhesion of bacteria to mucosal surfaces. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 35–48.
-
- Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- Cash H.L., Hooper L.V. Commensal bacteria shape intestinal immune system development. ASM News. 2005;71:77–83.
-
- Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. - PubMed
-
- Savage D.G. Mucosal microbiota. In: Mestecky J., Bienenstock J., Lamm M.E., Mayer L., McGhee J.R., Strober W., editors. Mucosal Immunology. 3rd ed. Elsevier/Academic Press; Amsterdam: 2005. pp. 19–33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous