Chaperone-mediated Cu+ delivery to Cu+ transport ATPases: requirement of nucleotide binding
- PMID: 19525226
- PMCID: PMC2742845
- DOI: 10.1074/jbc.M109.016329
Chaperone-mediated Cu+ delivery to Cu+ transport ATPases: requirement of nucleotide binding
Abstract
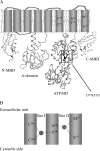
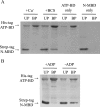
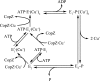
Cu(+)-ATPases drive the efflux of Cu(+) from the cell cytoplasm. During their catalytic/transport cycle, cytoplasmic Cu(+)-chaperones deliver the metal to the two transmembrane metal-binding sites (TM-MBSs) responsible for Cu(+) translocation. Here, using Archaeoglobus fulgidus Cu(+)-ATPase CopA and the C-terminal Cu(+)-chaperone domain of CopZ (Ct-CopZ), we describe the mechanism of Cu(+) transfer to both TM-MBSs. In absence of other ligands, Ct-CopZ transfers Cu(+) to wild-type CopA and to various CopA constructs lacking or having mutated cytoplasmic metal-binding domains, in a fashion consistent with occupancy of a single TM-MBS. Similar experiments performed in the presence of 2.5 mm ADP-Mg(2+), stabilizing an E1.ADP, lead to full occupancy of both TM-MBSs. In both cases, the transfer is largely stoichiometric, i.e. equimolar amounts of Ct-CopZ.Cu(+) saturated the TM-MBSs. Experiments performed with CopA mutants lacking either TM-MBS showed that both sites are loaded independently, and nucleotide binding does not affect their availability. The nucleotide-induced E2-->E1 transition is structurally characterized by a large displacement of the A and N domains opening the cytoplasmic region of P-type ATPases. Then, it is apparent that, whereas the first Cu(+)-chaperone can bind an ATPase form available in the absence of ligands, the second requires the E1.nucleotide intermediary to interact and deliver the metal. Interestingly, independent of TM-MBS Cu(+) loading, nucleotide binding also prevents the regulatory interaction of the N-terminal cytoplasmic metal-binding domain with the nucleotide binding domain.
Figures

References
-
- Fraústro da Silva J. J. R., Williams R. J. P. (2001) The Biological Chemistry of the Elements, 2nd Ed., pp. 418–435, Oxford University Press, New York
-
- Argüello J. M., Eren E., González-Guerrero M. (2007) Biometals 20, 233–248 - PubMed
-
- Kim B.E., Nevitt T., Thiele D. J. (2008) Nat. Chem. Biol. 4, 176–185 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

