Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells
- PMID: 19525400
- PMCID: PMC2695754
- DOI: 10.1073/pnas.0901418106
Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells
Abstract
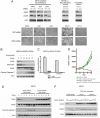
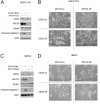
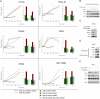
Two genes have a synthetically lethal relationship when the silencing or inhibiting of 1 gene is only lethal in the context of a mutation or activation of the second gene. This situation offers an attractive therapeutic strategy, as inhibition of such a gene will only trigger cell death in tumor cells with an activated second oncogene but spare normal cells without activation of the second oncogene. Here we present evidence that CDK2 is synthetically lethal to neuroblastoma cells with MYCN amplification and over-expression. Neuroblastomas are childhood tumors with an often lethal outcome. Twenty percent of the tumors have MYCN amplification, and these tumors are ultimately refractory to any therapy. Targeted silencing of CDK2 by 3 RNA interference techniques induced apoptosis in MYCN-amplified neuroblastoma cell lines, but not in MYCN single copy cells. Silencing of MYCN abrogated this apoptotic response in MYCN-amplified cells. Inversely, silencing of CDK2 in MYCN single copy cells did not trigger apoptosis, unless a MYCN transgene was activated. The MYCN induced apoptosis after CDK2 silencing was accompanied by nuclear stabilization of P53, and mRNA profiling showed up-regulation of P53 target genes. Silencing of P53 rescued the cells from MYCN-driven apoptosis. The synthetic lethality of CDK2 silencing in MYCN activated neuroblastoma cells can also be triggered by inhibition of CDK2 with a small molecule drug. Treatment of neuroblastoma cells with roscovitine, a CDK inhibitor, at clinically achievable concentrations induced MYCN-dependent apoptosis. The synthetically lethal relationship between CDK2 and MYCN indicates CDK2 inhibitors as potential MYCN-selective cancer therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma.Clin Cancer Res. 2015 Nov 15;21(22):5100-9. doi: 10.1158/1078-0432.CCR-15-0313. Epub 2015 Jul 22. Clin Cancer Res. 2015. PMID: 26202950 Free PMC article.
-
CDK4 inhibition restores G(1)-S arrest in MYCN-amplified neuroblastoma cells in the context of doxorubicin-induced DNA damage.Cell Cycle. 2013 Apr 1;12(7):1091-104. doi: 10.4161/cc.24091. Epub 2013 Mar 5. Cell Cycle. 2013. PMID: 23462184 Free PMC article.
-
CDK/CK1 inhibitors roscovitine and CR8 downregulate amplified MYCN in neuroblastoma cells.Oncogene. 2014 Dec 11;33(50):5675-87. doi: 10.1038/onc.2013.513. Epub 2013 Dec 9. Oncogene. 2014. PMID: 24317512 Free PMC article.
-
MDM2 as MYCN transcriptional target: implications for neuroblastoma pathogenesis.Cancer Lett. 2005 Oct 18;228(1-2):21-7. doi: 10.1016/j.canlet.2005.01.050. Cancer Lett. 2005. PMID: 15927364 Review.
-
MYCN oncoprotein targets and their therapeutic potential.Cancer Lett. 2010 Jul 28;293(2):144-57. doi: 10.1016/j.canlet.2010.01.015. Epub 2010 Feb 13. Cancer Lett. 2010. PMID: 20153925 Review.
Cited by
-
LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression.Nat Genet. 2012 Nov;44(11):1199-206. doi: 10.1038/ng.2436. Epub 2012 Oct 7. Nat Genet. 2012. PMID: 23042116
-
Inhibition of checkpoint kinase 1 following gemcitabine-mediated S phase arrest results in CDC7- and CDK2-dependent replication catastrophe.J Biol Chem. 2019 Feb 8;294(6):1763-1778. doi: 10.1074/jbc.RA118.005231. Epub 2018 Dec 20. J Biol Chem. 2019. PMID: 30573684 Free PMC article.
-
Myc mediates cancer stem-like cells and EMT changes in triple negative breast cancers cells.PLoS One. 2017 Aug 17;12(8):e0183578. doi: 10.1371/journal.pone.0183578. eCollection 2017. PLoS One. 2017. PMID: 28817737 Free PMC article.
-
Cyclin-dependent protein kinases and cell cycle regulation in biology and disease.Signal Transduct Target Ther. 2025 Jan 13;10(1):11. doi: 10.1038/s41392-024-02080-z. Signal Transduct Target Ther. 2025. PMID: 39800748 Free PMC article. Review.
-
Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma.Clin Cancer Res. 2015 Nov 15;21(22):5100-9. doi: 10.1158/1078-0432.CCR-15-0313. Epub 2015 Jul 22. Clin Cancer Res. 2015. PMID: 26202950 Free PMC article.
References
-
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. - PubMed
-
- Friend SH, Oliff A. Emerging uses for genomic information in drug discovery. N Engl J Med. 1998;338:125–126. - PubMed
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. - PubMed
-
- van Noesel MM, Versteeg R. Pediatric neuroblastomas: Genetic and epigenetic ‘danse macabre’. Gene. 2004;325:1–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

