Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues
- PMID: 19525403
- PMCID: PMC2747682
- DOI: 10.1096/fj.09-135467
Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues
Erratum in
- FASEB J. 2010 Feb;24(2):648
Abstract
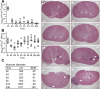
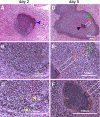
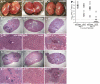
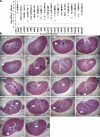
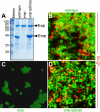
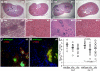
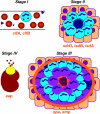
Staphylococcus aureus infections are associated with abscess formation and bacterial persistence; however, the genes that enable this lifestyle are not known. We show here that following intravenous infection of mice, S. aureus disseminates rapidly into organ tissues and elicits abscess lesions that develop over weeks but cannot be cleared by the host. Staphylococci grow as communities at the center of abscess lesions and are enclosed by pseudocapsules, separating the pathogen from immune cells. By testing insertional variants in genes for cell wall-anchored surface proteins, we are able to infer the stage at which these molecules function. Fibrinogen-binding proteins ClfA and ClfB are required during the early phase of staphylococcal dissemination. The heme scavenging factors IsdA and IsdB, as well as SdrD and protein A, are necessary for abscess formation. Envelope-associated proteins, Emp and Eap, are either required for abscess formation or contribute to persistence. Fluorescence microscopy revealed Eap deposition within the pseudocapsule, whereas Emp was localized within staphylococcal abscess communities. Antibodies directed against envelope-associated proteins generated vaccine protection against staphylococcal abscess formation. Thus, staphylococci employ envelope proteins at discrete stages of a developmental program that enables abscess formation and bacterial persistence in host tissues.
Figures


References
-
- Wallenfang T, Bohl J, Kretzschmar K. Evolution of brain abscess in cats formation of capsule and resolution of brain edema. Neurosurg Rev. 1980;3:101–111. - PubMed
-
- Russell D G. Staphylococcus and the healing power of pus. Cell Host Microbe. 2008;3:115–116. - PubMed
-
- Klevens R M, Morrison M A, Nadle J, Petit S, Gershman K, Ray S, Harrison L H, Lynfield R, Dumyati G, Townes J M, Craig A S, Zell E R, Fosheim G E, McDougal L K, Carey R B, Fridkin S K, Investigators for the Active Bacterial Core surveillance (ABCs) MRSA Investigators Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

