Identification and quantification of glycoproteins using ion-pairing normal-phase liquid chromatography and mass spectrometry
- PMID: 19525481
- PMCID: PMC2742440
- DOI: 10.1074/mcp.M900088-MCP200
Identification and quantification of glycoproteins using ion-pairing normal-phase liquid chromatography and mass spectrometry
Abstract
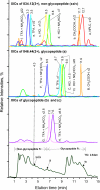
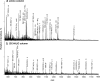
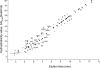
Glycoprotein structure determination and quantification by MS requires efficient isolation of glycopeptides from a proteolytic digest of complex protein mixtures. Here we describe that the use of acids as ion-pairing reagents in normal-phase chromatography (IP-NPLC) considerably increases the hydrophobicity differences between non-glycopeptides and glycopeptides, thereby resulting in the reproducible isolation of N-linked high mannose type and sialylated glycopeptides from the tryptic digest of a ribonuclease B and fetuin mixture. The elution order of non-glycopeptides relative to glycopeptides in IP-NPLC is predictable by their hydrophobicity values calculated using the Wimley-White water/octanol hydrophobicity scale. O-linked glycopeptides can be efficiently isolated from fetuin tryptic digests using IP-NPLC when N-glycans are first removed with PNGase. IP-NPLC recovers close to 100% of bacterial N-linked glycopeptides modified with non-sialylated heptasaccharides from tryptic digests of periplasmic protein extracts from Campylobacter jejuni 11168 and its pglD mutant. Label-free nano-flow reversed-phase LC-MS is used for quantification of differentially expressed glycopeptides from the C. jejuni wild-type and pglD mutant followed by identification of these glycoproteins using multiple stage tandem MS. This method further confirms the acetyltransferase activity of PglD and demonstrates for the first time that heptasaccharides containing monoacetylated bacillosamine are transferred to proteins in both the wild-type and mutant strains. We believe that IP-NPLC will be a useful tool for quantitative glycoproteomics.
Figures

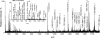



References
-
- Jaeken J., Matthijs G. (2001) Congenital disorders of glycosylation. Annu. Rev. Genomics Hum. Genet 2, 129–151 - PubMed
-
- Arnold J. N., Wormald M. R., Sim R. B., Rudd P. M., Dwek R. A. (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol 25, 21–50 - PubMed
-
- Rudd P. M., Elliott T., Cresswell P., Wilson I. A., Dwek R. A. (2001) Glycosylation and the immune system. Science 291, 2370–2376 - PubMed
-
- Varki A., Cummings R., Esko J., Freeze H., Marth J., Hindsgaul O., Paulson J., Lowe J., Arbo A., Manzi A., Powell L. (1999) Essentials of Glycobiology Cold Spring Harbor Laboratory Press, NY - PubMed
-
- Ferrara N., Kerbel R. S. (2005) Angiogenesis as a therapeutic target. Nature 438, 967–974 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

