Receptive fields and functional architecture in the retina
- PMID: 19525561
- PMCID: PMC2718235
- DOI: 10.1113/jphysiol.2009.170704
Receptive fields and functional architecture in the retina
Abstract
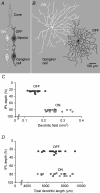
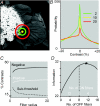
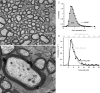
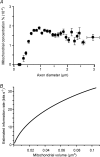
Functional architecture of the striate cortex is known mostly at the tissue level--how neurons of different function distribute across its depth and surface on a scale of millimetres. But explanations for its design--why it is just so--need to be addressed at the synaptic level, a much finer scale where the basic description is still lacking. Functional architecture of the retina is known from the scale of millimetres down to nanometres, so we have sought explanations for various aspects of its design. Here we review several aspects of the retina's functional architecture and find that all seem governed by a single principle: represent the most information for the least cost in space and energy. Specifically: (i) why are OFF ganglion cells more numerous than ON cells? Because natural scenes contain more negative than positive contrasts, and the retina matches its neural resources to represent them equally well; (ii) why do ganglion cells of a given type overlap their dendrites to achieve 3-fold coverage? Because this maximizes total information represented by the array--balancing signal-to-noise improvement against increased redundancy; (iii) why do ganglion cells form multiple arrays? Because this allows most information to be sent at lower rates, decreasing the space and energy costs for sending a given amount of information. This broad principle, operating at higher levels, probably contributes to the brain's immense computational efficiency.
Figures





References
-
- Ahmad KM, Klug K, Herr S, Sterling P, Schein S. Cell density ratios in a foveal patch in macaque ratina. Vis Neurosci. 2003;20:189–209. - PubMed
-
- Atick JJ, Redlich AN. What does the retina know about natural scenes? Neural Comput. 1992;4:196–210.
-
- Attwell D, Laughlin S. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Balasubramanian V, Berry MJ. A test of metabolically efficient coding in the retina. Network. 2002;13:531–552. - PubMed
-
- Balasubramanian V, Kimber D, Berry MJ. Metabolically efficient information processing. Neural Comput. 2001;13:799–815. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

